Development of Environmentally-Benign Cross-Coupling Reactions
Transition-metal-catalyzed cross-coupling reactions have been recognized as highly useful tools for the syntheses of pi-conjugated molecules. However, the conventional cross-coupling method has the following weak points: 1) the formation of stoichiometric amounts of salt-wastes is usually involved and 2) starting materials such as aromatic halides and arylmetal reagents are not always readily available. This work targets the development of environmentally-benign, waste-free dehydrogenative cross-coupling reactions of widely available aromatic hydrocarbons with unsaturated compounds under air. New catalyst systems consisting of rhodium or ruthenium complexes for the dehydrogenative cross-coupling have already been developed.
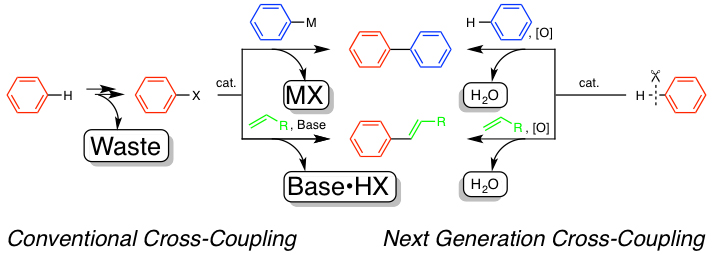
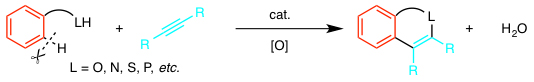
Recently, oxygen, nitrogen, and other heteroatom-containing substituents (–LH) have been found to act as directing groups to induce chelation-assisted C–H bond cleavage. They allow smooth coupling at the neighboring position. Especially in the coupling with alkynes, a variety of fused heterocyclic compounds can be constructed in a single step.
Organic Synthesis Using Readily Available Building Blocks
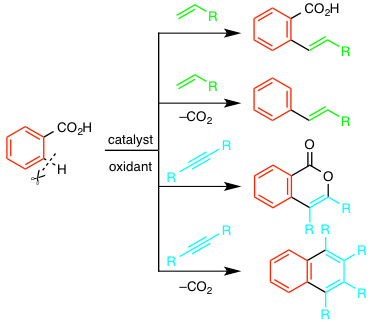
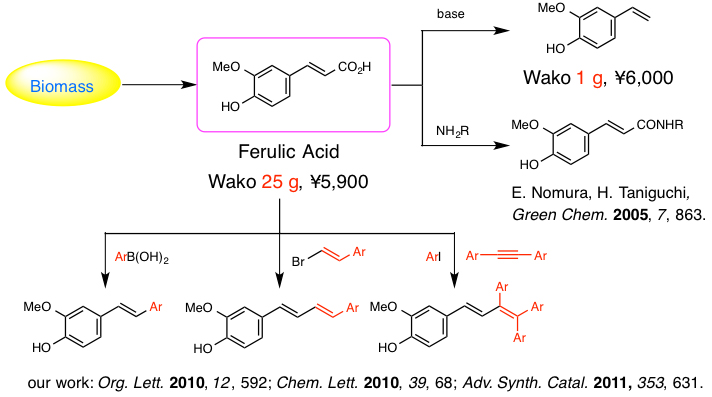
Fossil resources including petroleum, coal, and natural gas are currently predominant raw materials for the production of chemicals in modern society. However, in the near future, we have to develop the chemicals feeding system based on renewable resources such as biomass. The change of feedstock requires new organic synthetic methods. We now focus on carboxylic acids as readily available building blocks from biomass. A number of new reactions enabling the efficient transformation of carboxylic acids to various functionalized molecules have been developed by our group.
Antibiotics with Interesting Biological Activities
Microbial species have a capability to produce a wide variety of bioactive compounds with novel structures and various biological activities. Since UK-2A, an antimycin class antibiotic, was first isolated in 1996 from a soil sample collected at Sugimoto campus, we have been engaged in studies toward synthesis and biological evaluation of di/tri/tetra lactone antibiotics. 1) Splenocins were isolated from organic extract of marine-derived Streptomyces strain CNQ431 as potent anti-inflammatory antibiotics in 2009, which displayed low nanomolar activity in the suppression of cytokine production by OVA stimulated splenocytes. The structures of splenocins are similar to those of antimycin A3 and UK-2A.
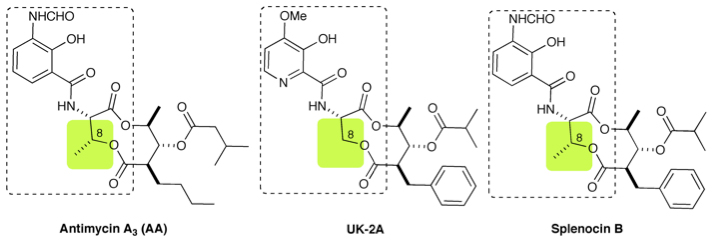
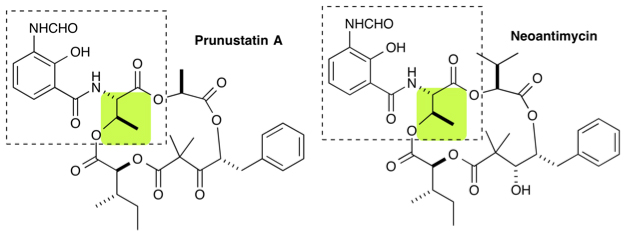
2) Neoantimycin is a rare and unusual ring-extended member of the antimycin class. First isolated in 1967 from a South American soil isolate of Streptomyces orinoci. Literature references were limited, but the recent discovery of prunustatin A as a selective GRP78 molecular chaperone down-regulator, which could lead to the development of new approaches toward combatting cancer, highlights the potential of this class as research probes.
Fluorinated Biomimetics
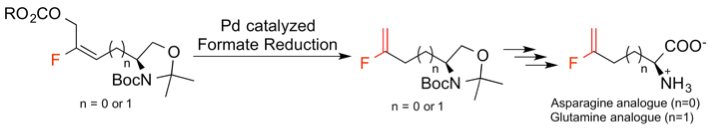
The ability of fluorine to impart unique properties to organic molecules has been exploited in the design of fluorine-containing bioactive compounds. Functionalized fluoroolefins are particularly important, with current applications in the synthesis of biologically active materials such as peptide isosteres. In our continuing studies on synthetic organofluorine chemistry towards fluorinated biomimetics, we have been interested in replacement of side-chain amide moieties of asparagine and glutamine with fluoroolefins, which are proposed to be aprotic mimic for amides due to their electronic properties. Asparagine and glutamine residues play important structural roles in proteins because their side-chain amide groups could act as both hydrogen bond acceptors and donors. We have developed a new access to fluorine-containg asparagine and glutamine analogues via formate reduction.