1. Current Research and Principal Research
Interests
(1) Molecular programming controlling supramolecular complex helicity:
Helical chirality in natural supramolecular systems
is well controlled by some external stimuli, and offers several
biological events such as gene expression and cellular processes. For
example, right-handed DNA changes its helical sense to left-handed one
(Z-DNA), caused by specific external stimuli. Several
artificial systems including polyacetylenes, polysilanes and
oligopeptides were reported to offer helicity inversion by chiral or
achiral stimuli. A variety of helicated metal complexes have been
recently developed, in which geometry of the metal center can determine
the supramolecular helical structure. Although these previous examples
included the kinetically inert metal centers and rendered the inversion
processes slow and incomplete, I recently demonstrated that the
kinetically labile Co(II) complex allowed dynamic and efficient
helicity inversion as required for supramolecular switching devices.
This helicity invertible complex can induce one-handed helicity in oligopeptides and work as a time-tunable unit in time scale from milliseconds to hours.
“Coordination Chemistry Strategies for Dynamic Helicates: Time-Programmable Chirality Switching with Labile and Inert Metal Helicates”,
Chem. Soc. Rev., 41, 6977-6991 (2012).
“Helicity Inversion from Left- to Right-Handed Square Planar Pd(II) Complexes: Synthesis of a Diastereomer Pair from a Single Chiral Ligand and their Structure Dynamism”,
Chem. Commun., 48, 3721-3723 (2012).
“Time-Programmed Peptide Helix Inversion of Synthetic Metal Complex Triggered by Achiral NO3- Anion”,
J. Am. Chem. Soc., 130, 792-793 (2008).
“Dynamic Helicity Inversion by Achiral Anion Stimulus in Synthetic Labile Cobalt(II) Complexes”,
J. Am. Chem. Soc., 126,6524-6525 (2004).
(Highlighted by Science, 304, 1215, (2004), Editor’s Choice).
(2) Model
studies of active sites in
metalloproteins: Transition metal ions in numerous metalloproteins
play important roles in a variety of functions including hydroxylation
of methane, the generation of amino acid radicals, hydrolysis of
phosphate esters, and oxygen storage and transport. My object in this
area is to gain fundamental structural, spectroscopic, and mechanistic
understanding of those transition metal ions in active site of protein
environments via the synthesis, characterization, and examination of
reactivity of model
complexes. These studies led me to the production of a novel catalyst
which
should work in an ambient condition without producing poisonous
materials
as byproducts. In collaboration with Prof. Lawrence Que, Jr.
(University
of Minnesota), I studied the oxidation abilities of high valent
Fe(IV)=O
species in a non-heme iron complex. My current studies with synthetic
models
focus on the relationship between stereoselective oxidation activity
and
configurational environment around the high valent metal center.
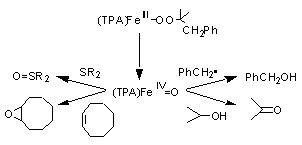
“‘Intermolecular’
Trapping of a Nonheme Fe(IV)=O Intermediate”, Inorg.
Chem., 40 , 3534-3538(2001).
“Evidence for a Nonheme Fe(IV)=O Species in the Intramolecular
Hydroxylation of a Phenyl Moiety”, J. Am. Chem. Soc., 121,
6330-6331(1999).
(3) Functional studies of rare earth metal complexes: Another
of my interests is the field of complexation of rare earth metal ions
by optically active chelates. Because the rare earth metal ion has a
number of coordination sites, the resulting complexes with hexa -
octadentate ligand or three bidentate ligand still have one or more binding sites in which
bidentate ligand such as amino acids or monodentate ligand such as
water molecules as the relatively weak third ligand can bind to the ion center.
On the basis of the magnetic, optical and chiroptical properties of rare earth metal ions,
such complexes are used as chiral shift reagents in
NMR studies, as contrast enhancers in magnetic resonance imaging and as chiral luminescence and sensing devices.
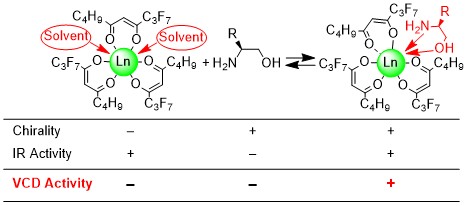
“Lanthanide Tris(β-diketonates) as Useful Probes for Chirality Determination of Biological Amino Alcohols in Vibrational Circular Dichroism: Ligand to Ligand Chirality Transfer in Lanthanide Coordination Sphere”,
Chirality, 26, 293-299 (2014).
“Molecular Recognition and Sensing via Rare Earth Complexes”,
Handbook on the Physics and Chemistry of Rare Earths,
35, Chapter 226, (2005), (Elsevier, Amsterdam).
“Novel Optically-active Bis(amino acid) Ligands and Their Complexation with
Gadolinium” J. Chem. Soc., Dalton Trans., 1119-1125(2002).
“Europium(III) - N,N’-Ethylenebis(L-amino acid) Complexes as
New Chiral NMR Lanthanide Shift Reagents for Unprotected α-Amino Acids
in Neutral Aqueous Solution”, Bull. Chem. Soc. Jpn., 74,
707-715(2001).
While the synthesis of new molecules lies at the center of my research
effort, I also use a wide array of techniques to characterize the
compounds I prepare and to examine their reactivity. Among the
characterization methods I use an X-ray crystallography, NMR, EPR,
UV-Vis, FTIR, fluorescence, Circular Dichroism (CD) and
resonance Raman spectroscopy, mass spectrometry, GC/MS,
potentiometry and cyclic voltammetry. I also
endeavor to unravel reaction mechanisms through kinetics and isotope
labeling experiments and to examine reaction selectivity through GC and
HPLC methods. Students in the group thus obtain a highly
multidisciplinary training in the synthesis, structural and
spectroscopic characterization, and mechanistic study of organic and
inorganic molecules.
2. Selected Publications
Original Papers:
1. “Lanthanide Tris(β-diketonates) as Useful Probes for Chirality Determination of Biological Amino Alcohols in Vibrational Circular Dichroism: Ligand to Ligand Chirality Transfer in Lanthanide Coordination Sphere”,
H. Miyake, K. Terada and H. Tsukube,
Chirality, 26, 293-299 (2014).
2. “Helicity Inversion from Left- to Right-Handed Square Planar Pd(II) Complexes: Synthesis of a Diastereomer Pair from a Single Chiral Ligand and their Structure Dynamism”,
H. Miyake, M. Ueda, S. Murota, H. Sugimoto and H. Tsukube,
Chem. Commun., 48, 3721-3723 (2012).
3. “Time-Programmed Peptide Helix Inversion of Synthetic Metal Complex Triggered by Achiral NO3- Anion”,
H. Miyake, H. Kamon, I. Miyahara, H. Sugimoto and H. Tsukube,
J. Am. Chem. Soc., 130, 792-793 (2008).
4. “A Chemical Device That Exhibits Dual Mode Motions: Dynamic Coupling of Amide Coordination Isomerism and Metal-Centered Helicity Inversion in a Chiral Cobalt(II) Complex”,
H. Miyake, M. Hikita, M. Itazaki, H. Nakazawa, H. Sugimoto and H. Tsukube,
Chem. Eur. J., 14, 5393-5396 (2008).
5. “Dynamic Helicity Inversion by Achiral Anion Stimulus in Synthetic
Labile Cobalt(II) Complexes”, H. Miyake, K. Yoshida, H. Sugimoto
and H. Tsukube, J. Am. Chem. Soc., 126,
6524-6525 (2004). (Highlighted by Science,
304, 1215, (2004), Editor’s Choice).
6. “Novel Optically-active Bis(amino acid) ligands and Their
Complexation with Gadolinium”, H. Miyake, M. Watanabe, M.
Takemura, T. Hasegawa, Y. Kojima, M. B. Inoue, M. Inoue and Q.
Fernando, J. Chem. Soc., Dalton Trans., 1119-1125 (2002).
Review Articles:
7. “Supramolecular Chirality in Dynamic Coordination Chemistry”,
H. Miyake,
Symmetry, 6, 880-895 (2014).
8. “Materials-Based Receptors: Design Principle and Applications”,
K. Singh, D. Sareen, P. Kaur, H. Miyake and H. Tsukube,
Chem. Eur. J., 19, 6914-6936 (2013).
9. “Coordination Chemistry Strategies for Dynamic Helicates: Time-Programmable Chirality Switching with Labile and Inert Metal Helicates”,
H. Miyake and Hiroshi Tsukube,
Chem. Soc. Rev., 41, 6977-6991 (2012).
Books:
10. ”Supramolecular Chemistry Strategies for Naked-Eye Detection and Sensing”
K. Singh, P. Kaur, H. Miyake and H. Tsukube,
Synergy in Supramolecular Chemistry, (eds) T. Nabeshima, CRC Press, (2014).
|