|
|
|
|
Laboratory of Bio-Functionl Molecular Design
|
|
|
Hiroshi Nakajima
(Professor)
|
|
|
|
1. Current Research and
Principal Research Interests
We have focused on
development of novel biomaterials based on coordination and electro-
chemistry, which include artificial metallo-enzymes,
stimuli responsible nano-carriers, electrochemical signal transduction with
proteins and so on. Characterization of a novel signal transducer protein,
particularly a transcriptional regulator, is also a target in our research,
aiming at the exploitation of its functional mechanism to a future signal
transduction system. In light of the practical application of proteins as a
durable molecular material, we are interested in the functional
transformation of proteins from thermophiles by making use of genetic techniques
and chemical modification. The followings are some research projects on
going in our research group.
|
Application of a signal transducer protein
as a sensing module of electrochemical bio-sensor. A transcriptional regulator
is a member of signal transducer proteins which specifically sense an
environmental stress to treat it by regulating activity of appropriate
proteins at the transcriptional level. In biological systems, various
transcriptional regulators have evolved to respond to all kinds of
stresses not only chemical substances but also physical stimuli such as
light, heat, and osmotic pressure. In general, the stress detection by
the transcriptional regulators is highly sensitive and specific, which
are essential to eliminate the stresses and retain homeostasis of living
cells. Meanwhile, these properties appear attractive in light of
constructing a novel bio-based sensor device. In order to realize such
biosensor, we are focusing to construct an electrochemical system to
transduce the readout of a transcriptional regulator to an electronic
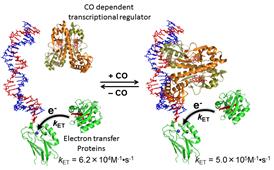
signal. The figure below shows a recent achievement in this research
project. For more details, please visit the home page of our research
group.
|
|
Alteration in the binding affinity of
CO-dependent transcriptional regulator (the intrinsic readout of the
regulator) is transduced to modulation in the electron transfer rate
between two electron transfer proteins.
|
|
|
Cytohrome c552 from Thermus thermophirus.
A frequently used protein in our research project
|
Creation of thermally
tolerant peroxidase transforming a protein from thermophilic bacterium Proteins are generally considered
to be fragile under conditions other than room temperature and near
neutral aqueous solution and readily lose their functions. This is because proteins familiar to us
derive from cells that work in the environment moderate to our
cells. Proteins produced in
microorganisms which grow (as viewed from us) in harsh environments,
however, has excellent properties to be tolerant
to the environment. Using such
proteins, we are trying to create an artificial enzyme and nano-carrier
which shows sufficient thermal stability required on practical use. For more details, please
visit the home page of our research group.
|
|
Characterization
of Transcriptional regulator of nitrogenase, another pathway to
understand nitrogenase In present days, artificial nitrogen fixation (reduction of
nitrogen in the air to ammonia) reaches approximately to 10% of whole
ecosystem (Ref. 1), which is essential for the production activities of the
human race. Most of them have been demanded by the Haber process. In
light of energy saving for material production, development of
alternative processes that do not require high temperature and high
pressure would become a more and more important issue. Among several
challenges to realize such processes, use of bacteria that produce
nitrogenase (a nitrogen-fixing enzyme) as biomass is counted to be a
strong candidate. However, there are several big problems before putting
it into practical use. One of them is to understand what growing
environment maximizes ability of bacterial nitrogen fixation.
|
|
|
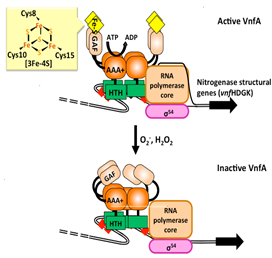
Detailed characterization of the nitrogenase would be a
conventional method to understand suitable conditions to maximize the
nitrogenase function. Meanwhile, our have taken another approach to do
so: functional characterization of the transcriptional regulator of
nitrogenase. Recent achievements are schematized in the left-hand. For more details, please
visit the home page of our research group.
|
|
|
|
|
2. Selected Publications
1. Miura Y., Yoshimitsu
K., Takatani N., Watanabe Y., Nakajima H., Effect
of Nitric Oxide on VnfA, a transcriptional activator of nitrogenase-2, in Azotobacter
vinelandii. J. Biochem. 157, 365-375, 2015.
2. Rabindra KB., Nakajima H., Jitumani R., Watanabe Y.,
Mazumdar S., Thermodynamic effects of the alteration of the axial ligand on
the unfolding of the thermostable cytochrome c. Biochemistry, 52, 1373-1384, 2013.
3. Ibrahim Sk. Md., Nakajima H., Ramanathan
K., Takatani N., Ohta T., Naruta
Y., Watanabe Y., Cytochrome c552
from Thermus Thermophilus
Engineered for Facile Conversion of the Prosthetic Group. Biochemistry, 50, 9826-9835, 2011.
4. Nakajima H., Takatani N., Yoshimitsu K., Itoh M., Aono S., Takahashi Y., Watanabe
Y., The role of Fe-S cluster in the sensory domain of nitrogenase
transcriptional activator VnfA Azotobacter
vinelandii. FEBS J., 277, 817-832, 2010.
5. Rosenberger N., Studer A., Takatani N., Nakajima H., Watanabe Y., Azurin–Poly(N-isopropylacrylamide) Conjugates by Site-Directed
Mutagenesis and their Temperature Dependent Behavior in Electron Transfer
Processes. Angew. Chem. Int. Ed.,
48, 1946-1949, 2009.
6. Nakajima H., Ichikawa Y., Satake
Y., Takatani N., Manna SK., Rajbongshi J.,
Mazumdar S., Watanabe Y., Engineering of Thermus thermophilus Cytochrome c552: Thermally Tolerant
Artificial Peroxidase. ChemBioChem, 9, 2954-2957,
2008.
7. Tokita Y., Shimura J., Nakajima H., Goto Y., Watanabe Y.,
Mechanism of Intermolecular electron Transfer in the Photoexcited
Zn-Substituted Cytochrome c: Theoretical and Experimental Perspective. J. Am. Chem. Soc., 130, 5302-5310, 2008.
8. Satake Y., Abe S., Okazaki S., Ban
N., Hikage T., Ueno T., Nakajima H., Suzuki A.,
Yamane T., Nishiyama H., and Watanabe Y.,
Incorporation of a phebox rhodium complex into apo-myoglobin affords a stable organometallic protein
showing unprecedented arrangement of the complex in the cavity. Organometallics, 26, 4904-4908, 2007.
9. Watanabe Y., Nakajima H., Ueno T., Reactivities
of oxo and peroxo
intermediates studied by hemoprotein mutants. Acc Chem
Res, 40, 554-562, 2007.
10. Ichikawa Y., Nakajima H., Watanabe Y., Characterization of
peroxide bound heme species generated in reaction of thermally tolerant
cytochrome c552 with
hydrogen peroxide. ChemBioChem, 7, 1582-1589,
2006.
|
|
|