1. Current
Research and Principal Research Interests
Our principal
research interests include total synthesis, structural elucidation,
biosynthesis, and mechanism of action for biologically active and
structurally unique natural products, asymmetric synthesis,
organometallic chemistry, reaction mechanism, and conformational
analysis.
(1)
Total Synthesis and Complete Assignment of the Stereostructures
of Squalene-Derived Cytotoxic Triterpene Polyethers (Oxasqualenoids)
Total Synthesis and Complete Assignment of
the Stereostructure of a Cytotoxic Bromotriterpene Polyether
(+)-Aurilol: The plane structure and partial stereochemistry of
a cytotoxic bromotriterpene polyether (+)-aurilol (1), isolated from the sea hare Dolabella auricularia, were mainly
elucidated by NMR methods; however, determination of the entire
stereochemistry has not been reached. Although there have also been
many other types of triterpene polyethers, it is often difficult to
determine their stereostructures even by the current highly advanced
spectroscopic methods, especially in acyclic systems including
quaternary carbon centers such as C10–C11, C14–C15, and C18–C19 in 1. In this paper, we report that the
total assignment of the incomplete stereostructure of (+)-aurilol (1) to the structural formula 2 has been accomplished through its
first asymmetric total synthesis featuring the highly regio- and
stereocontrolled biogenetic-like A–D ether ring formations.

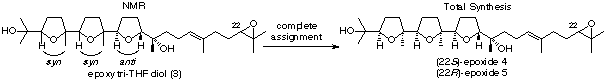
Complete Assignment of the Stereostructure
of a New Squalene-Derived Epoxy Tri-THF Diol from Spathelia glabrescens by Total
Synthesis: The total assignment of the incomplete
stereostructure of a new squalene-derived epoxy tri-tetrahydrofuran
(THF) diol (3) to the
structural formula 4 has been
achieved through the first asymmetric syntheses of (22S)-4
and its epimer (22R)-5.
Total Synthesis and Determination of the
Absolute Configuration of (–)-Longilene Peroxide: The first
asymmetric total synthesis of (–)-longilene peroxide (6) has been achieved starting from
the optically active C2-symmeric
diepoxide through the concept of two-directional synthesis utilizing
its intrinsic molecular symmetry. Thus, the unknown absolute
configuration of longilene peroxide has been determined by this
synthesis as shown in the structural formula 6.
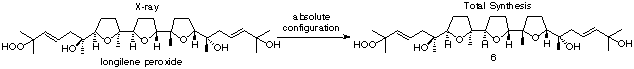
(2)
Total Synthesis of Biologically Active and Structurally
Interesting Nitrogen-Containing Natural Products
Total
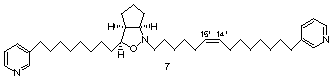
Synthesis and
Assignment of the Double-Bond Position and Absolute Configuration of
(–)-Pyrinodemin A: The first asymmetric total synthesis of a
structurally novel cis-cyclopent[c]isoxazolidine
alkaloid, (–)-pyrinodemin A (7),
which exhibits potent cytotoxicity, has been accomplished through a
highly diastereoselective intramolecular nitrone-olefin cycloaddition
reaction as the key step. Thus, it has been found that the hitherto
unknown absolute configuration of pyrinodemin A is as indicated in the
structural formula 7.
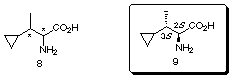
Total Synthesis and Determination of the
Stereochemistry of 2-Amino-3-cyclopropylbutanoic Acid, a Novel Plant
Growth Regulator Isolated from the Mushroom Amanita castanopsidis Hongo: The
unknown stereostructure of 2-amino-3-cyclopropylbutanoic acid 8, a novel plant growth regulator
isolated from the mushroom Amanita castanopsidis Hongo, was determined
to be (2S,3S)-9
through its racemic and enantioselective syntheses employing the
chelate-enolate Claisen rearrangement as a key step.
Stereocontrolled Total Synthesis of the
Stemona Alkaloid (–)-Stenine: The Stemona alkaloid stenine (10), isolated from Stemona tuberosa of physiologically
active stemonaceous plants, possesses the structurally novel and unique
azepinoindole skeleton (B,C,D-ring system). We have accomplished the
asymmetric total synthesis of (–)-stenine (10), starting from 1,5-pentanediol.
The key features are an intramolecular diastereoselective Diels-Alder
reaction of the (E,E,E)-triene
11, prepared in a
convergent fashion from three readily available compounds, and
efficient construction of the tricyclic A,B,D-ring system through
thermodynamically controlled regioselective enolization of the bicyclic
ketone 12.
2.
Selected Publications
1.
“Reagent-Controlled Switching of 5-Exo to 6-Endo Cyclizations in
Epoxide Openings”, Y.
Morimoto, Y. Nishikawa, C. Ueba, and T. Tanaka, Angew. Chem., Int. Ed., in press.
2. “Structures, Biological Activities, and Total Syntheses of
13-Hydroxy- and 13-Acetoxy-14-nordehydrocacalohastine, Novel Modified
Furanoeremophilane-Type Sesquiterpenes from Trichilia cuneata”, M. Doe, T.
Shibue, H. Haraguchi, and Y.
Morimoto, Org.
Lett., 7, 1765–1768 (2005).
3. “Total Synthesis and Complete Assignment of the Stereostructure of a
Cytotoxic Bromotriterpene Polyether (+)-Aurilol”, Y. Morimoto, Y. Nishikawa,
and M. Takaishi, J.
Am. Chem. Soc., 127, 5806–5807 (2005).
4. “Total Synthesis and Assignment of the Double-Bond Position and
Absolute Configuration of (–)-Pyrinodemin A”, Y. Morimoto, S. Kitao, T.
Okita, and T. Shoji, Org. Lett., 5,
2611–2614 (2003).
5. “Total Synthesis of (+)-Eurylene and (+)-14-Deacetyl Eurylene”, Y. Morimoto, K. Muragaki,
T. Iwai, Y. Morishita, and T. Kinoshita, Angew. Chem., Int. Ed., 39,
4082–4084 (2000).
6. “Revised Structure of Squalene-Derived PentaTHF Polyether,
Glabrescol, through Its Enantioselective Total Synthesis:
Biogenetically Intriguing CS
vs C2
Symmetric Relationships”, Y.
Morimoto, T. Iwai, and T. Kinoshita, J. Am. Chem. Soc., 122, 7124–7125 (2000).
7. “Can α-Sultone Exist as a
Chemical Species? First Experimental Implication for Intermediacy of α-Sultone”, Y. Morimoto, H. Kurihara,
and T. Kinoshita, Chem. Commun.,
189–190 (2000).
8. “Effective Combination of Two-Directional Synthesis and Rhenium(VII)
Chemistry: Total Synthesis of meso
Polyether Teurilene”, Y.
Morimoto, T. Iwai, and
T. Kinoshita, J. Am. Chem. Soc.,
121, 6792–6797
(1999).
9. “Highly Diastereoselective Cylizations of Bishomoallylic Tertiary
Alcohols Promoted by Rhenium(VII) Oxide. Critical Steric versus
Chelation Effects in Alkoxyrhenium Intermediates”, Y. Morimoto and T. Iwai, J. Am. Chem. Soc., 120, 1633–1634 (1998).
10. “Studies on the Asymmetric Synthesis of Stemona Alkaloids: Total
Synthesis of (–)-Stenine”, Y.
Morimoto, M. Iwahashi, K. Nishida, Y. Hayashi, and H. Shirahama,
Angew. Chem., Int. Ed.
Engl., 35, 904–906
(1996).
|