1. My Most Current Research and
Principal Research Interests
My research interests include the total synthesis of
bioactive natural products, its biological activities, the structure-activity relationship
study and new synthesis method for the total synthesis.
(1) Total Synthesis of 10-Isocyano-4-cadinene
and Its Stereoisomers and Evaluations of Antifouling Activities
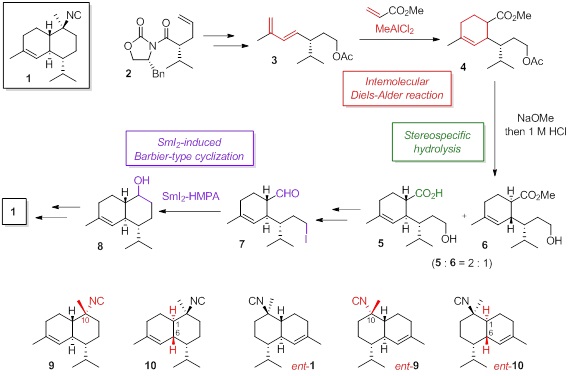
@@10-Isocyano-4-cadinene (1), a marine
sesquiterpene isolated from nudibranchs of the family Phyllidiidae by Okino et al.,
exhibits potent antifouling activity against the larvae of the barnacle Balanus amphitrite (EC50 0.14
Ęg/mL). 1 is expected to be new nontoxic antifouling agents.
Furthermore, the absolute stereochemistry of 1 has not been determined.
To access these issues, we started the enantioselective total synthesis toward 1.
@@@The
synthesis toward 1 was commenced
with the known imide 2, which was
converted into the dieneacetate 3.
The Diels-Alder reaction between 3
and methyl acrylate with MeAlCl2 afforded cyclohexene 4 as a mixture of four diasereomers.
This mixture was epimerized to two isomers, the desired carboxylic acid 5 as a major product and the ester 6, by the treatment of NaOMe followed
by selective hydrolysis with 1 M HCl in one-pot. The carboxylic acid 5 was transformed into the aldehyde 7, which was cyclized by SmI2-initiated
Barbier-type reaction to provide the alcohol 8. At last, the total synthesis of 1 was achieved via the
introduction of the functional groups at C10. The absolute configuration
of 1 was determined as (1S, 6S,
7R, 10S) by comparison of the optical rotations between natural and
synthetic samples. In addition, I successfully synthesized 10-epi-10-isocyano-4-cadinene (9), di-1,6-epi-10-isocyano-4-cadinene (10)
and its enantiomers (ent-1, ent-9 and ent-10) through the same
synthetic pathway. Antifouling activities against Balanus amphitrite with the cadinenes were also evaluated.
(2) Confirmation
of the Configuration of 10-Isothiocyanato-4-cadinene Diastereomers
Through Synthesis
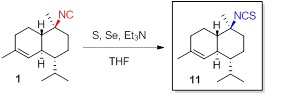
@@The marine sponge metabolite 10-isothiocyanato-4-cadinene (11) was first isolated by Garson et al. from Acanthella
cavernosa in 2000. The same structure 11
was later reported by Wright from the nudibranch Phyllidiella pustulosa and its sponge diet, but with different
NMR data. The syntheses of both enantiomers of 11 were accomplished through the isothiocyanation of (+)-10-isocyano-4-cadinene
(1) and (-)-1. The
correct spectroscopic data and specific
rotation value of the structure 11
were determined on the basis of the syntheses. The NMR data of synthetic 11 matched those of the isothiocyanate
isolated by Garson and differed
from those reported by Wright.
(3) Total
Synthesis of Stemonamine Using Ynolate
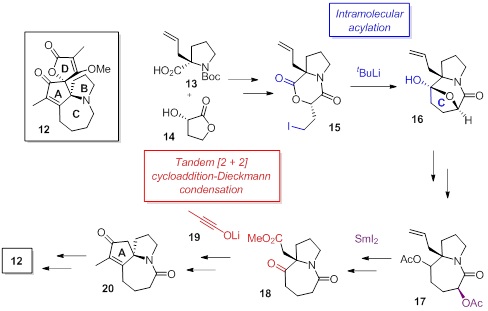
@@@Stemonamine (12) was isolated from the roots of Stemona japonica Miq. as a member of the Stemona alkaloid family. Although the racemic total synthesis of 12 has been reported by three groups, its
enantioselective synthesis has not been achieved so far. In this work, the
total synthesis of 12 using two key reactions, the ynolate-initiated tandem reaction and the
intramolecular acylation, is described.
@@Our
total synthesis commenced with condensation of the known optically active carboxylic
acid 13 and the lactone 14 to give the iodide 15. The intramolecular acylation of 15 using tBuLi afforded the 7-membered ring 16 in high yield. The NaBH4 reduction of 16 followed by acetylation provided the
diacetate 17. After the removal of
the acetoxy group on the C-ring via
the SmI2-mediated reduction, the obtained monoacetate was
transformed to the g-ketoester 18.
As the second key step, the tandem [2+2] cycloaddition-Dieckmann condensation
using the ynolate 19 and 18 was performed to successfully
provide the tricyclic compound 20, the ABC-ring system of 12, in high yield. The total
synthesis of 12 was achieved via the known construction method of
the D-ring. The spectroscopic data of our synthetic 12 were in good agreement with those recorded in the literature.
(4) The Present Research
@@@In the present
work, the asymmetric total
syntheses of (-)-lepadiformine A (21) and (-)-histrionicotoxin (22) are now ongoing.
2.
Selected Publications
1. g Confirmation
of the configuration of 10-isothiocyano-4-cadinene diastereomers through synthesis
h K. Nishikawa, T. Umezawa, M. J. Garson, F. Matsuda J. Nat. Prod. 75, 2232?2235 (2012).
2. g Key
structural features of cis-cinnamic
acid as an allelochemical h M. Abe, K. Nishikawa,
H. Fukuda, K. Nakanishi, Y. Tazawa, T. Taniguchi, S.-Y. Park, S. Hiradate, Y.
Fujii, K. Okuda, M. Shindo Phytochemistry
84, 56?67 (2012).
3. g Stereoselective synthesis of b-glycosyl
esters of cis-cinnamic acid and its
derivatives using unprotected glycosyl donors h K. Matsuo, K.
Nishikawa, M. Shindo Tetrahedron Lett. 52, 5688?5692
(2011).
4. g Total synthesis of 10-isocyano-4-cadinene and its
stereoisomers and evaluations of antifouling activities h K.
Nishikawa, H. Nakahara, Y. Shirokura, Y. Nogata,
E. Yoshimura, T. Umezawa, T. Okino, F. Matsuda J. Org.
Chem. 76, 6558?6573 (2011).
5. g Total synthesis of
10-isocyano-4-cadinene and determination of its absolute configuration h K.
Nishikawa, H. Nakahara, Y. Shirokura, Y. Nogata, E. Yoshimura,
T. Umezawa, T. Okino, F. Matsuda Org. Lett. 12,
904?907
(2010).
|