1. Current Research and Principal Research InterestsMy major research interest is the development of new methodology for the
synthesis of biologically important optically active compound and/or silicon-containing
functional compound using chiral α-hydroxysilane. We have established
the gram scale preparation of α-hydroxysilanes in optically pure form
(>95% ee). I am also interested in a synthetic use of unprecedented
α-silyl cationic species, which can be generated from α-hydroxysilane.
(1) Chirality transferring CC bond formation using [3,3] sigmatropic rearrangement of α-acyloxyallylsilane
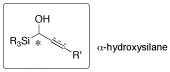
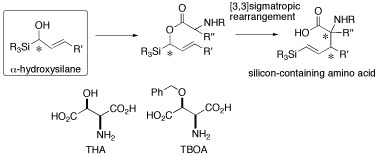
Optically active α-hydroxyallylsilane has attracted significant interest in view of its potential utility in a diastereoselective functionalization of its allylic CC double bond. A vinylsilane-containing α-amino acid and α,α-disubstituted α-amino acid having two contiguous asymmetric carbon centers at their α and β positions were synthesized in an optically active form by ester-enolate Claisen rearrangement of the α-acyloxyallylsilane. This proved that the α-hydroxysilylane is an excellent chirality transferring group in a [3,3] sigmatropic rearrangement. Recently, as an application of this methodology, we synthesized four types of optically active analogs of threo-β-hydroxy aspartate (THA) and an analog of threo-β-benzyloxy aspartate (TBOA), which are potent blockers of excitatory amino acid transporters in the mammalian central nervous system. Conformational studies of these analogs by NMR gave important suggestions toward the active conformation of TBOA.
(2) Synthesis of biologically active natural compound
Amathaspiramide F, an alkaloid isolated from New Zealand marine byrozoan, possesses a unique structure characterized by contiguous asymmetric carbon centers on its spirobicyclic core. We planned its total synthesis using the chirality transferring ester-enolate Claisen rearrangement of an α-acyloxykllylsilane as a key step. 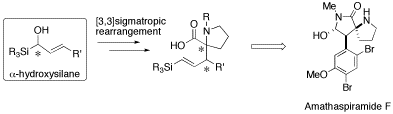
(3) Systematic study of α-silyl cationic species and its synthetic application
We reported that acidic treatment of optically active α-hydroxycyclopropylsilane gave several rearranged products via hitherto unprecedented α-silyl cation where the optical purity of the starting α-hydroxysilane was completely retained. Recently, we performed systematic studies of the acid-catalyzed reaction of alkyl, alkenyl or alkynyl substituted α-hydroxysilane, and found their reactivity profile. We also succeeded in inter- and intramolecular palladium-catalyzed allylic alkylations of optically active α-acyloxyallylsilanes. These reactions proceeded in a regio- and stereoselective manner via α-silyl substituted π-allyl palladium complex, which is regarded as a synthetic equivalent to α- and γ-silyl cation species, to give the corresponding (E)-vinylsilanes in which the ee of the starting material was completely transferred to the product. 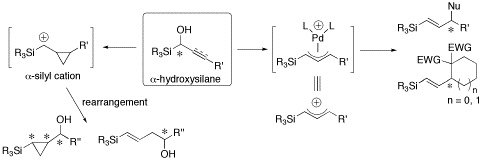
2. Selected Publications
1. Palladium-catalyzed allylic alkylation of optically active α-alkenyl-α-acyloxytrialkylsilane. K. Sakaguchi, T. Yamada, Y. Ohfune, Tetrahedron Lett. 2005, 46, 5009-5012.
2. Synthesis of Optically Active β-Alkyl Aspartate via [3,3] Sigmatropic Rearrangement of α-Acyloxytrialkylsilane. K. Sakaguchi, M. Yamamoto, T. Kawamoto, T. Yamada, T. Shinada, K. Shimamoto, Y. Ohfune, Tetrahedron Lett. 2004, 45, 5869-5872.
3. Acid-Catalyzed Rearrangement of α-Hydroxytrialkylsilanes. K. Sakaguchi, M. Higashino, and Y. Ohfune, Tetrahedron 2003, 59, 6647-6658.
4. Chirality Transferring [3,3] Sigmatropic Rearrangement of (1-Acyloxy-2-alkenyl)trialkylsilane. Synthesis of Optically Active Vinylsilane-Containing α-Amino Acid. K. Sakaguchi, H. Suzuki, and Y. Ohfune, Chirality 2001, 13, 357-365.
5. Reverse Brook Rearrangement of 2-Alkynyl Trialkylsilyl Ether. Synthesis of Optically Active (1-Hydroxy-2-alkynyl)trialkylsilane. K. Sakaguchi, M. Fujita, H. Suzuki, M. Higashino, and Y. Ohfune, Tetrahedron Lett. 2000, 41, 6589-6592.
6. Syntheses of Optically Active 2-Substituted Cyclopropanecarboxylic Acids from Chiral α-Hydroxysilane Derivatives. K. Sakaguchi, H. Mano, and Y. Ohfune, Tetrahedron Lett. 1998, 39, 4311-4312.
7. Acid-Catalyzed Rearrangement of α-Hydroxycyclopropylsilanes. K. Sakaguchi, M. Fujita, and Y. Ohfune, Tetrahedron Lett. 1998, 39, 4313-4316.
8. "Pd-C Assisted Synthesis of cis-2,4-Disubstituted-γ-Butyrolactones and their Properties for Ferroelectric Liquid Crystals", K. Sakaguchi, Y. Kawamura, S. Saito, and Y. Ohfune, Synlett, 1997, 624-626.
9. Synthesis of Both Enantiomers of Halitunal. K. Shimano, Y. Ge, K. Sakaguchi, and S. Isoe, Tetrahedron Lett. 1996, 37, 2253-2256.
10. Synthesis and Properties of Optically Active α-Aryl-γ-alkyl-γ-lactones as Chiral Dopants for Ferroelectric Liquid Crystals. K. Sakaguchi, T. Kitamura, Y. Shiomi, M. Koden, and T. Kuratate, Chem. Lett. 1991, 1383-1386.
|