1. Current Research and Principal Research Interests
My research interests are directed into (1) total synthesis of biologically active natural products, (2) synthesis of novel squaric acid-containing amino acid isostere and its incorporation into biologically active peptides, and
(3) isolation of the venom components of insects and their evaluation of the biological functions.
(1) Total synthesis of biologically active natural products
Nitrogen atom-containing natural products are rich source of the biologically active compounds.
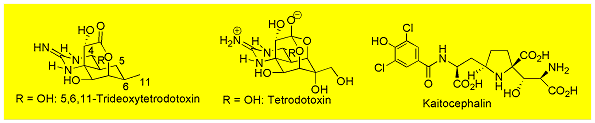
We are interested in total synthesis of tetrodotoxin and its congeners, kaitocephalin, and manzacidines, known as sodium channel blockers, AMPA/KA receptor antagonist, and adrenoceptor receptor blocker, respectively.
The key to the total synthesis relies on the use of our original synthetic methodology, asymmetric transferring Strecker synthesis, for the construction of the quaternary amino carbon centers commonly found in this class of natural products.
As a result, we have furnished total synthesis of 5,6,11-trideoxy tetrodotoxin, kaitocephalin and its 7-isomer, and manzacidine A~C and its isomers.
Biological evaluations of these natural products as well as total synthesis of tetrodotoxin are in progress.
(2) Synthesis of novel squaric acid-containing amino acid isostere and its incorporation into biologically active peptides.
Squaric acid is know as a carboxylic surrogate and widely utilized for the area of medicinal chemistry, bioconjugate chemistry, material science, and organic chemistry.
We have interesting in the multifunctional properties, e.g., acidity, aromaticity, UV absorption, electrophilic reactivity for nucleophiles, and hydrogen-bond formations, and planned synthesis of novel amino acid surrogate bearing squaric acid.
To this end, we developed a novel synthetic approach for formal substitution of the carboxylate to squaric acid via the decarboxylation of a squarylacetate. We also have established the incorporation of the amino acid analogs into biologically active peptides (Leu-Enkephalin, an opioid peptide).
This is the first example of synthesis and incorporation of amino acid analogs bearing squaric acid. Further bioorganic studies using novel squaric acid containing amino acids are in progress.
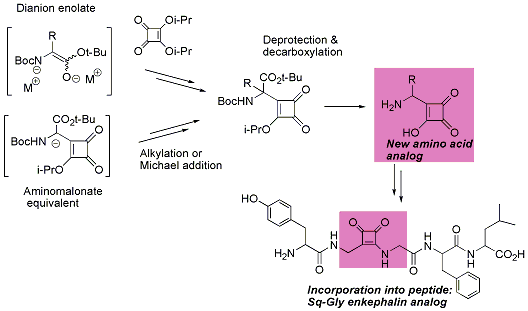
(3) Isolation of the venom components of insects and their evaluation of the biological functions.
The venom of insects and arthropods abundantly includes biologically active compounds.
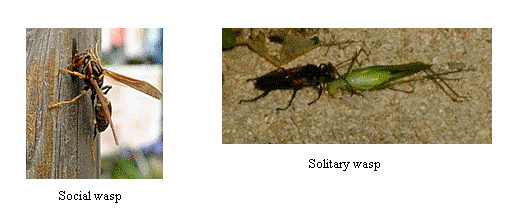
We recently examined the venom component of the venom of social and solitary wasps.
In the venom of social wasps, we found novel peptides exhibiting histamine-releasing and hemolytic activities, named Polistes mastoparan-R1 to R3, and Polistes protonectin
that exhibite more potent biological activities than those of peptides previously isolated from the venom of yellow jackets and hornets.
Solitary wasps are known as hunting wasps. Although they use the venom, the purpose differs from the case of social wasps. Solitary wasps use the venom to paralyze the other insects.
The venom of solitary wasps has received much attention as their intriguing biological activities to induce long-term paralysis of the preys since famous French entomologist, J.-H. Fabre was reported the interesting phenomena. We investigated the venom using various kinds of highly sensitive analytical methodologies, e.g. MALDI-TOF, ESI-Q-TOF, CE, CE-MS, NMR, ICP-MS. As a result, we clarified the key major venom components that were able to induce the long-term paralysis by their synergic effects.
1. "Asymmetric synthesis of α,α-disubstituted amino acids", Y. Ohfune and T. Shinada,
Eur. J. Org. Chem., 2005, in press.
2. "Synthesis of α-Amino Squaric Acids Using an Aminomalonate Equivalent Bearing Squaryl Group",
T. Shinada, T. Ishida, and Y. Ohfune, Synthesis, 2005, 2723-2729.
3. "Molecular components and toxicity of the venom of the solitary wasp, Anopplius samariensis",
M. Hisada, H. Satake, K. Masuda, M. Aoyama, K. Murata, T. Shinada,, T. Iwashita, Y. Ohfune, and T. Nakajima,
Biochem. Biophys. Res. Commun., 330, 1048-1054 (2005).
4. "Synthesis of novel amino squaric acids via addition of dianion enolates derived from N-Boc amino acid esters",
T. Ishida, T. Shinada, and Y. Ohfune, Tetrahedron Lett., 46, 311-314 (2005).
5. "Rapid and efficient identification of cysteine-rich peptides by random screening of a venom gland cDNA library from the hexathelid spider Macrothele gigas",
H. Satake, E. Villegasa, N. Oshiro, K. Terada, T. Shinada, and G. Corzo, Toxicon, 44, 149-156 (2004).
6. "Stereocontrolled synthesis of a potent agonist of Group II metabotropic glutamate receptors, (+)-LY354740, and its related derivatives",
Y. Ohfune, T. Demura, S. Iwama, H. Matsuda, K. Namba, K. Shimamoto, and T. Shinada, Tetrahedron Lett., 44, 5431-5434 (2003).
7. "Asymmetric Strecker Route toward the Synthesis of Biologically Active α,α-Disubstituted α-Amino Acids:"
Y. Ohfune, and T. Shinada,, Bull. Chem. Soc. Jpn., 76, 1115-1129 (2003).
8. "Syntheses and Paralytic Activities of Squaryl Amino Acid-Containing Polyamine Toxins",
T. Shinada, Y. Nakagawa, K. Hayashi, G. Corzo, T. Nakajima, and Y. Ohfune, Amino Acids, 24, 293-302 (2003).
9."Total Synthesis and Absolute Structure of Manzacidin A and C.",
K. Namba, T. Shinada, T. Teramoto, and Y. Ohfune, J. Am. Chem.
Soc. , 112, 10708-10709 (2000).
10. "Asymmetric Strecker Route toward the Corey Intermediate of Lactacystin.",
S. Iwama, W. Gao, T, Shinada, and Y. Ohfune, Synlett,
2000, 1631-1633.
|