1. Current Research and Principal Research Interests
(1) The quantum nature of light is applied to laser microscopy.
Since the miracle year of 1905 associated with Einstein, light has been intensely investigated to understand its quantum nature. In particular, many researchers have been interested in a microcavity as a tiny light resonator. Because the number of electromagnetic modes in the microcavity is limited, light-matter (atom or molecule) interaction is extremely modified in the microcavity. Such a modification is theoretically described by cavity quantum electrodynamics (cavity QED). In particular, spontaneous emission rate is enhanced or suppressed in the microcavity, known as the Purcell effect.
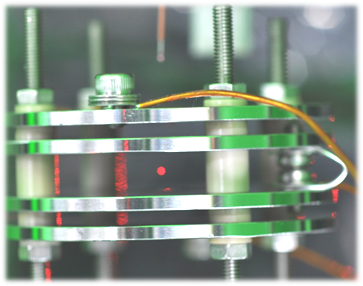 A levitated microdroplet in space can be regarded as an ideal microcavity having a huge quality factor (Q-factor) of more than 106, because the levitated microdroplet forms an almost perfect sphere due to its surface tension. Accordingly, the levitated microdroplet gives us a great opportunity of ultimate sensitive probe of biomacromolecules dissolved in the microdroplet, because they may show the Purcell effect and/or have the ultralow threshold for lasing. We have developed a single microdroplet levitation apparatus combined with a laser microscope to achieve ultimate biosensing by using the quantum nature of light.
(2) The nature of biomacromolecules is modified in physiological conditions.
One of the characteristic features of the living cells is its crowded macromolecular environment. While many biochemical experiments have been conducted in dilute solutions (in vitro) to understand biochemical processes such as protein folding and enzyme reactions, these do not adequately reflect the processes in vivo because macromolecular crowding in cells modifies the rates and the equilibria of the biochemical processes. In addition, such a crowded macromolecular environment may severely restrict the number of water molecules hydrating biomolecules, which may lead to the modifications of their functions. We have investigated the impact of macromolecular crowding on the structural stability and functions of biomacromolecules. 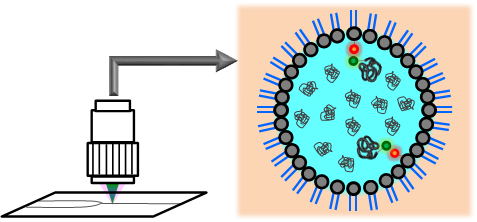
2. Selected Publications
1) "Macromolecular crowding modifies the impact of specific Hofmeister ions on the coil-globule transition of PNIPAM."
K. Sakota, D. Tabata, H. Sekiya, J. Phys. Chem. B, 119, 10334-10340 (2015).
2) "Weak hydrogen bonding motifs of ethylamino neurotransmitter radical cations in a hydrophobic environment: infrared spectra of tryptamine+-(N2)n clusters (n≤6)."
K. Sakota, M. Schuts, M. Schmies, R. Moritz, A. Bouchet, T. Ikeda, Y. Kouno, H. Sekiya, O. Dopfer, Phys. Chem. Chem. Phys., 16, 3798 (2014).
3) "Photoionization-induced water migration in the amide group of trans-acetanilide(H2O)1 in the gas phase."
K. Sakota, S. Harada, Y. Shimazaki, H. Sekiya, J. Phys. Chem. A, 115, 626-630, (2011).
4) "Excited-state triple-proton transfer in 7-azaindole(H2O)2 and reaction path studied by electronic spectroscopy in the gas phase and quantum chemical calculation."
K. Sakota, C. Jouvet. C. Dedonder, M. Fujii, H. Sekiya, J. Phys. Chem. A, 114, 11161-11166 (2010).
5) "Cooperativity of hydrogen-bonded networks in 7-azaindole(CH3OH)n (n=2,3) clusters evidenced by IR-UV ion-dip spectroscopy and natural bond orbital analysis."
K. Sakota, Y. Kageura, H. Sekiya, J. Chem. Phys. 129, 054303 (2008).
6) "Cooperative triple-proton/hydrogen atom relay in 7-azaindole(CH3OH)2 in the gas phase: Remarkable change in the reaction mechanism from vibrational-mode specific to statistical fashion with increasing internal energy."
K. Sakota, N. Inoue, Y. Komoto, H. Sekiya, J. Phys. Chem. A, 111, 4596-4603 (2007).
7) "Excited-state double-proton transfer in the 7-azaindole dimer in the gas phase. 3. Reaction mechanism studied by picosecond time-resolved REMPI spectroscopy."
K. Sakota, C. Okabe, N. Nishi and H. Sekiya, J. Phys. Chem. A, 109, 5245-5247 (2005).
|