1. My Most Current Research and Principal Research Interests
My current research interests are as follows. In order to obtain novel functionality magnetic materials based on the π-conjugated organic spin systems, we have investigated the novel organic compounds (π-electronic spin systems, organic high-spin molecules, stable radical or biradicals, organic cation or dications) with unpaired electrons. My most current interest is the novel photo-induced spin alignment utilizing the photo-excited spin state, which clarify the guiding principles of designing the novel photo-magnetic devices based on organic materials. These studies will be extended to construct novel magnetic devices, molecular memory or molecular quantum computer etc. based on organic materials. The details are described as follows.
The photoinduced spin alignment of π-conjugated organic spin systems.
My most current research interest is as follows. As a model system for the photo-induced spin alignment/photo-switched spin-conversion in the purely organic π-conjugated spin systems, 1 and 2 were designed and synthesized. Their photo-excited states and the photo-induced spin alignment were investigated by time-resolved electron spin resonance (TRESR). I made the first observation of the excited quartet (S = 3/2) spin state (Q) and the excited quintet (S = 2) state (Qu) on the π-conjugated spin
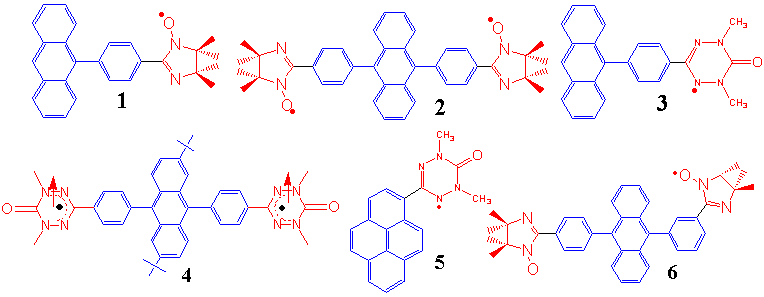
systems (π-conjugated triplet-radical pair systems). Since 2 shows a weak antiferromagnetic exchange interaction in the ground state, the clear detection of the excited quintet high-spin state shows that the effective exchange coupling between the two dangling radicals through the diphenylanthracene spin coupler has been changed from antiferromagnetic to ferromagnetic upon photo-excitation. Thus, a photo-induced spin alignment utilizing the excited triplet molecular field was realized for the first time in the purely organic π-conjugated spin system. In the mechanism of the photo-induced spin alignment, the role of the spin delocalization and the spin polarization mechanisms were revealed. High-spin excited states were not observed for the π-topological isomers of 1 and 2, showing the role of π-topology in the spin alignment of the excited states. This is the first systematic study of the π-topology and the spin alignment on the photo-excited states. Similar photo-induced spin alignment and the high-spin excited states were also observed for the phenyl- or diphenylanthracene-verdazyl (3 and 4) and pyrene-verdazyl radical (5) systems.
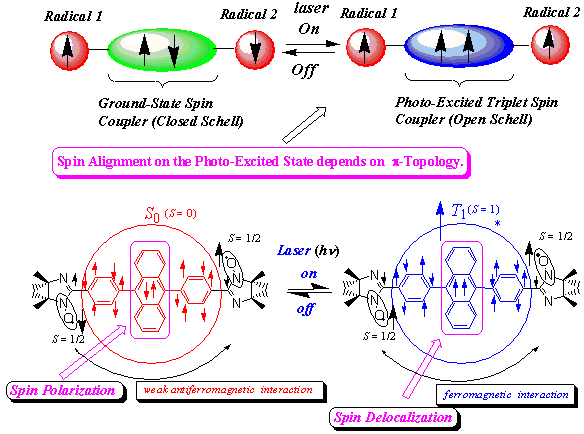
π-Conjugated spin systems have the following advantages in view point of the material science and molecular magnetism: (1) π-Conjugation leads to the strong exchange coupling which is one of the important conditions for the balk magnetism at finite temperature. (2) One can well design and synthesize the proper spin systems with the desired spin states using the well-established fine-synthetic chemistry. (3) An enhanced intersystem crossing (ISC) mechanism arising from the attachment of the radical species is available. This leads to open a way of the direct ESR detection of the silent photo-excited states with little ISC to the triplet state.
Very recently, a novel lowest photo-excited triplet state arising from four unpaired spins has been detected for the first time by the proper molecular designing taking π-topology into account. For the molecule 6, TRESR signals arising from the lowest triplet photo-excited state (T1) and the low-lying quintet spin state (Qu) closely above the triplet state were observed. The magnitude of the fine-structure parameter (D) of the triplet state is ca. 50% of that of anthracene. The unique triplet state has an interesting electronic structure, which D value is reduced by the antiferromagnetic spin alignment between two radical spins through the excited spin coupler. This small D value is characteristic nature of the unique triplet state, which is constructed from four unpaired electrons and explained well theoretically.
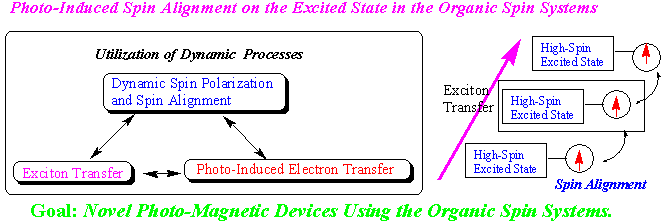
Photo-induced intermolecular spin alignment utilizing the excitonic process or photo-induced electron transfer are now investigating for the charge transfer (CT) crystals of π-conjugated spin systems using optical measurement, TRESR, and optically detected magnetic resonance (ODMR). Very recently, Pulsed-ESR apparatus combined with Nd:YAG laser has been set up and the study of the relaxation process are now going in progress. These studies will give the fundamental knowledge on the photo-magnetism based on the organic spin systems and lead to construction of novel magnetic devices, molecular memory or molecular quantum computer etc. ... based on organic materials.
2. Selected Publications
1. "π-Topology and Spin Alignment in Unique Photo-excited Triplet and Quintet Stated Arising from Four Unpaired Electrons of An Organic Spin System",
Y. Teki, T. Toichi and S. Nakajima,
Chem. Eur. J., 23, in press (2005).
2. "π-Topology and Spin Alignment Utilizing the Excited Molecular Field: Observation of
the Excited High-Spin Quartet (S = 3/2) and Quintet (S = 2) States on the Purely
Organic π-Conjugated Spin Systems",
Y. Teki, S. Miyamoto, M. Nakatsuji and Y. Miura,
J. Am. Chem. Soc., 123, 294-305 (2001).
3. "Intramolecular Spin Alignment Utilizing the Excited Molecular Field between the Triplet
(S = 1) Excited State and the Dangling Stable Radicals (S = 1/2) as Studied by
Time-Resolved Electron Spin Resonance: Observation of the Excited Quartet (S = 3/2) and
Quintet (S = 2) States on the Purely Organic π-Conjugated Spin Systems",
Y. Teki, S. Miyamoto, K. Iimura, M. Nakatsuji and Y. Miura,
J. Am. Chem. Soc., 122, 984-985 (2000).
4. "Design and Experimental Investigation of High-Spin Organic Systems",
Y. Teki and K. Itoh,
Magnetic Properties of Organic Materials, Ed. P. M. Lahti, Chapter 12, pp. 237-265, Marcel-Dekker, New-York, 1999.
5. "Magnetic Properties of Stable N-[(Dichlorophenyl)thio]-2,4,6-tri-phenylphenylaminyl and N-[(Dichlorophenyl)thio]-2,4,6-tris-(chlorophenyl)phenylaminyl Radical Crystals",
Y. Teki, K. Itoh, A. Okada, H. Yamakage, T. Kobayashi, A. Amaya, S. Kurokawa, S. Ueno and Y. Miura,
Chem. Phys. Lett., 270, 573-579 (1997).
6. "Topology and Spin Alignment in a Novel Organic High-Spin Molecule, Biphenyl-3,4'-bis(phenylmethylene), as Studied by ESR and a Generalized UHF Hubbard Calculation",
Y. Teki, I. Fujita, T. Takui, T. Kinoshita and K. Itoh,
J. Am. Chem. Soc., 116, 11499-11505 (1994).
7. "Novel Organic Ions of High-Spin States II.: Determination of the Spin Multiplicity of the Ground State and 1H-ENDOR Study of the of Monoanion of m-Phenylenebis-(phenylmethylene)",
M. Matsushita, T. Nakamura, T. Momose, T. Shida, (Kyoto Univ.) Y. Teki, T. Takui, T. Kinoshita, and K. Itoh, (Osaka City Univ.),
J. Am. Chem. Soc., 114, 7470-7475 (1992).
8. "Design, Preparation, and Electron Spin Resonance Detection of a Ground-State Undecet
(S=5) Hydrocarbon",
I. Fujita, Y. Teki, T. Takui, T. Kinoshita, K. Itoh, F. Miko, Y. Sawaki, H. Iwamura, A. Izuoka, and T. Sugawara,
J. Am. Chem. Soc., 112, 4074-4075 (1990).
9. "General Conditions for the Occurrence of Off-Axis Extra Lines in Powder-Pattern ESR Fine-Structure",
Y. Teki, T. Takui and K. Itoh,
J. Chem. Phys., 88, 6134-6145 (1988).
10. "Spin Alignment in Organic High-Spin Molecules. A Heisenberg Hamiltonian Approach I",
Y. Teki, T. Takui, M. Kitano and K. Itoh,
Chem. Phys. Lett., 142, 181-186 (1987).
11. "Preparation and ESR Detection of a Ground-State Nonet Hydrocarbon as a Model for One-Dimensional Organic Ferromagnets",
Y. Teki, T.Takui, K. Itoh, H. Iwamura and K. Kobayashi,
J. Am. Chem. Soc., 108, 2147-2156 (1986).
12. "Electron Spin Resonance Line-Shapes of Randomly Oriented Molecules in Septet and Nonet States by Perturbation Approach",
Y. Teki, T. Takui, H. Yagi, K. Itoh, and H. Iwamura,
J. Chem. Phys., 83, 539-547 (1985).
|