MORIMOTO RESERACH GROUP (SYNTHETIC ORGANIC CHEMISTRY LABORATORY)
TEL.06-6605-3141
〒558-8585 大阪府大阪市住吉区杉本3-3-138
発表活動PUBLICATIONS
SELECTED PUBLICATIONS ( Keisuke Nishikawa )
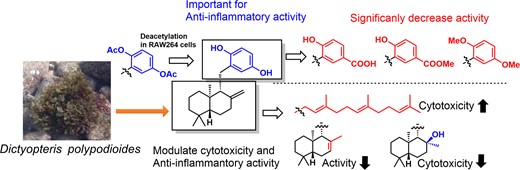
"Structure-Activity Relationship of Anti-Inflammatory Meroterpenoids Isolated from Dictyopteris polypodioides in RAW264 Cells" M. Kumagai, A. Matsuda, N. Shiiba, T. Tsuruta, H. Endo, K. Nishikawa, and Y. Morimoto Biosci. Biotech. Biochem. In Press (2024).ABSTRACT : In this study, we explored anti-inflammatory compounds from the brown alga Dictyopteris polypodioides and isolated 7 meroterpenoids. Their anti-inflammatory activities were evaluated using the lipopolysaccharide (LPS)-stimulated mouse macrophage cell line, RAW264. Yahazunol (1) exhibited similar nitric oxide (NO) production inhibitory activity as zonarol (2), which has previously been shown to be an anti-inflammatory compound. Yahazunol (1), zonarol (2), and isozonarol (3) inhibited not only nitric oxide production but also inducible nitric oxide synthase (iNOS), interleukin-6 (IL6), and C-C motif chemokine ligand 2 (CCL2) mRNA expression in RAW264 cells. The structure-activity relationships of the 11 compounds, including their synthetic analogs, revealed the significance of the hydroquinone moiety in the anti-inflammatory activity of these sesquiterpenoids in RAW264 cells. Diacetylated zonarol (9) exhibited an activity comparable to that of zonarol as a result of intracellular deacetylation. These results provide new insights into the anti-inflammatory activity of hydroquinone-containing natural products.
"Halogenated Cyclic Monoterpenoids with Anti-biofouling Activity from the Okinawan Red Marine Algae Portieria hornemannii" S. Ishigami, R. Fukada, G. Nagasaka, T. Tsuruta, K. Nishikawa, Y. Sasaki, K. Nimura, I. Oshima, Y. Yamagishi, Y. Morimoto, T. Kamada, and T. Ishii Chem. Biodivers. In Press (2024).
ABSTRCT : The red algal genus Portieria is a prolific producer of halogenated monoterpenoids. In this study, we isolated and characterised monoterpenoids from the Okinawan red algae Portieria hornemannii. A new polyhalogenated cyclic monoterpenoid, 2(R)-chloro-1,6(S)-dibromo-3(8)(Z)-ochtoden-4(R)-ol (1), along with three known monoterpenoids, (2R,3(8)E,4S,6R)-6-bromo-2-chloro-1,4-oxido-3(8)-ochtodene (2), 1-bromo-2-chloroochtoda-3(8),5-dien-4-one (3), and 2-chloro-1-hydroxyochtoda-3(8),5-dien-4-one (4) were isolated from the methanol extract of three populations of P. hornemannii. These compounds were characterised using a combination of spectroscopic methods and chemical synthesis, and the absolute stereochemistry of compounds 1 and 2 was determined. In addition, all isolated compounds were screened for their anti-biofouling activity against the mussel Mytilus galloprovincialis, and 1 exhibited strong activity. Therefore, halogenated monoterpenoids have the potential to be used as natural anti-biofouling drugs.
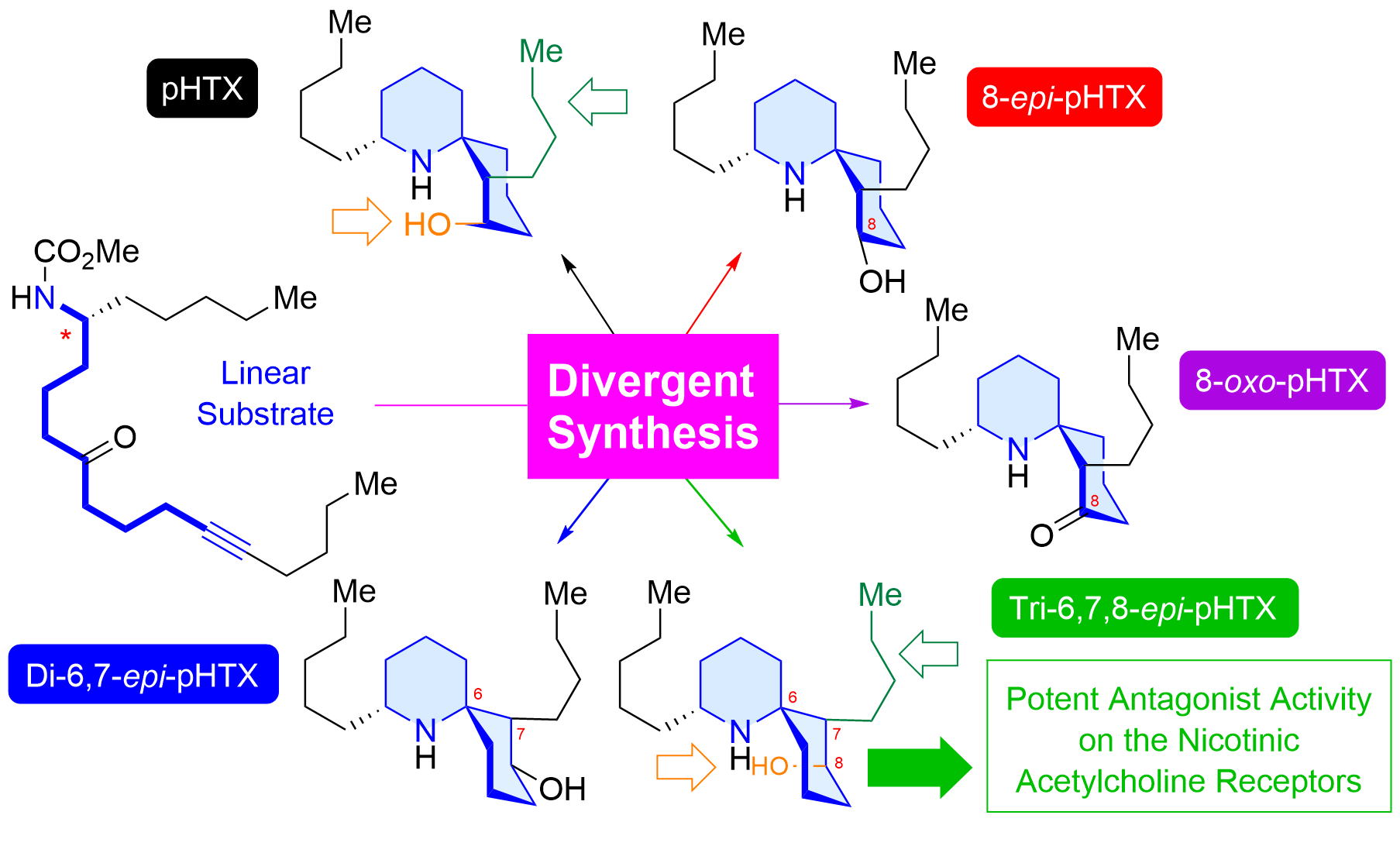
"Divergent Nine-Step Syntheses of Perhydrohistrionicotoxin Analogs and Their Inhibition Activity Toward Chicken α4β2-Neuronal Nicotinic Acetylcholine Receptors" K. Nishikawa, Y. Ono, S. Mori, K. Takayama, M. Ihara, K. Matsuda, and Y. Morimoto J. Org. Chem. 89, 4128-4133 (2024).
ABSTRACT : Histrionicotoxin (HTX) alkaloids, which are isolated from Colombian poison dart frogs, are analgesic neurotoxins that modulate nicotinic acetylcholine receptors (nAChRs) as antagonists. Perhydrohistrionicotoxin (pHTX) is the potent synthetic analogue of HTX and possesses a 1-azaspiro[5.5]undecane skeleton common to the HTX family. Here, we show for the first time the divergent nine-step synthesis of pHTX and its three stereoisomers from the known aldehyde through a one-step construction of the 1-azaspiro[5.5]undecane framework from a linear amino ynone substrate. Surprisingly, some pHTX diastereomers exhibited antagonistic activities on the chicken α4β2-neuronal nAChRs that were more potent than pHTX.
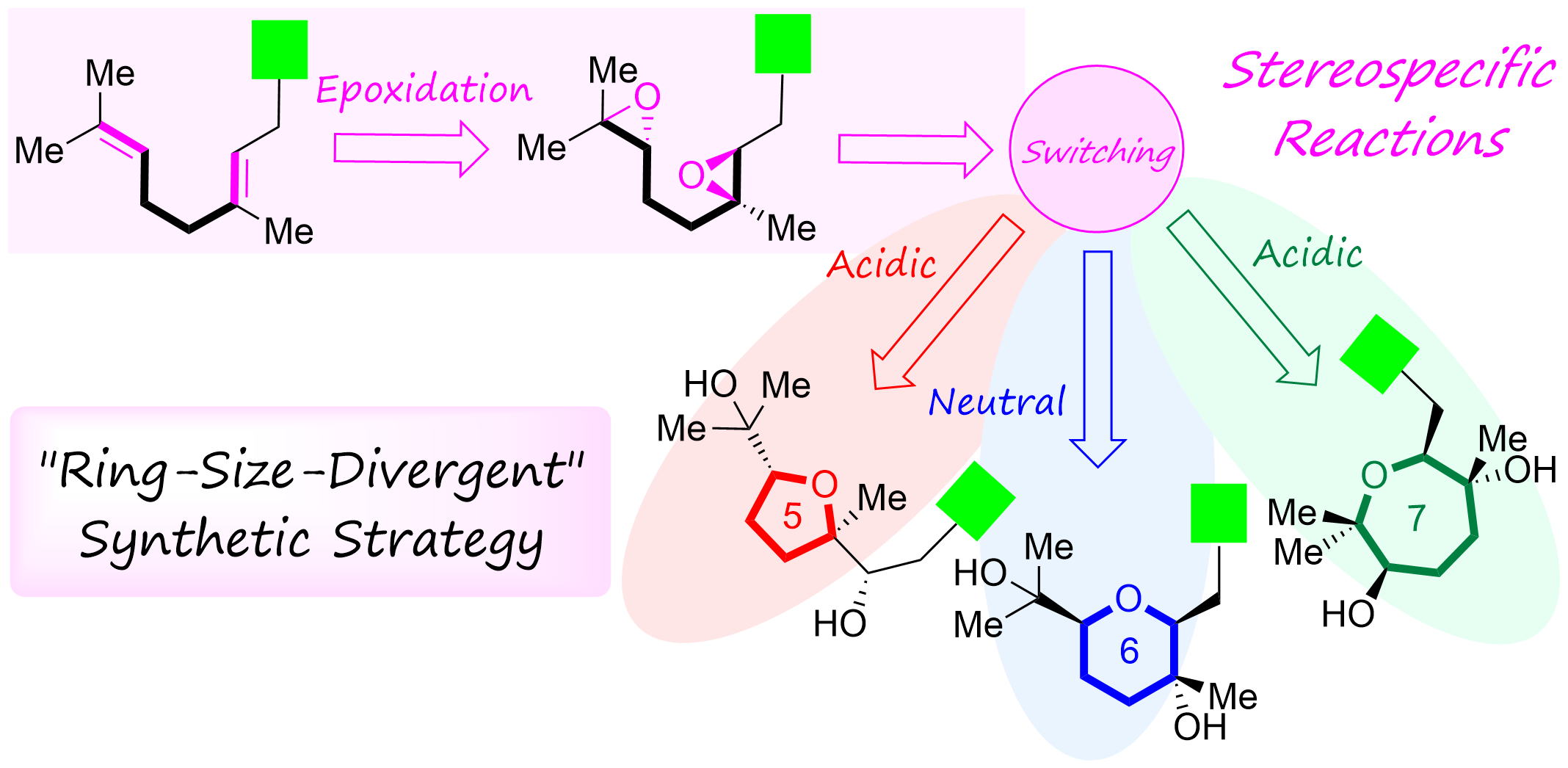
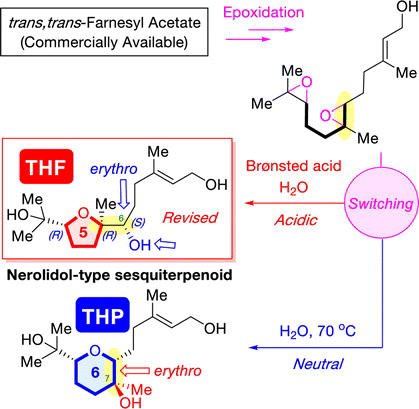
"Establishment of “Ring-Size-Divergent” Synthetic Strategy: Divergent Synthesis, Stereochemical Assignments, and Biological Activity Studies of Nerolidol-Type Sesquiterpenoids and Feroniellins" K. Nishikawa, T. Teranishi, T. Tsuruta, T. Niwa, K. Morita, S. Hashimoto, A. Hoshino, M. Kumagai, and Y. Morimoto J. Org. Chem. 88, 15844-15861 (2023).
ABSTRCT: Biomimetic epoxide-opening cascade cyclizations of polyepoxides enable the efficient and rapid construction of polyether skeletons. In this study, we discovered a method for switching the cyclization mode from tetrahydrofuran to tetrahydropyran (THP) formation in epoxide-opening cascades of polyepoxides. The THP formation proceeded via an epoxonium-ion intermediate by simple heating in neutral water. Next, by expanding the switching reaction, we successfully established a “ring-size-divergent” synthetic strategy that enabled the synthesis of the five-, six-, and seven-membered ether rings from identical diepoxide cyclization precursors under simple acidic or neutral conditions. The “ring-size-divergent” synthetic strategy was applied to the short divergent synthesis of nerolidol-type sesquiterpenoids and feroniellins, resulting in the revision of the proposed stereochemistry of certain natural products and the determination of all of the absolute configurations. Additionally, the anti-inflammatory activities of the synthetic samples were evaluated.
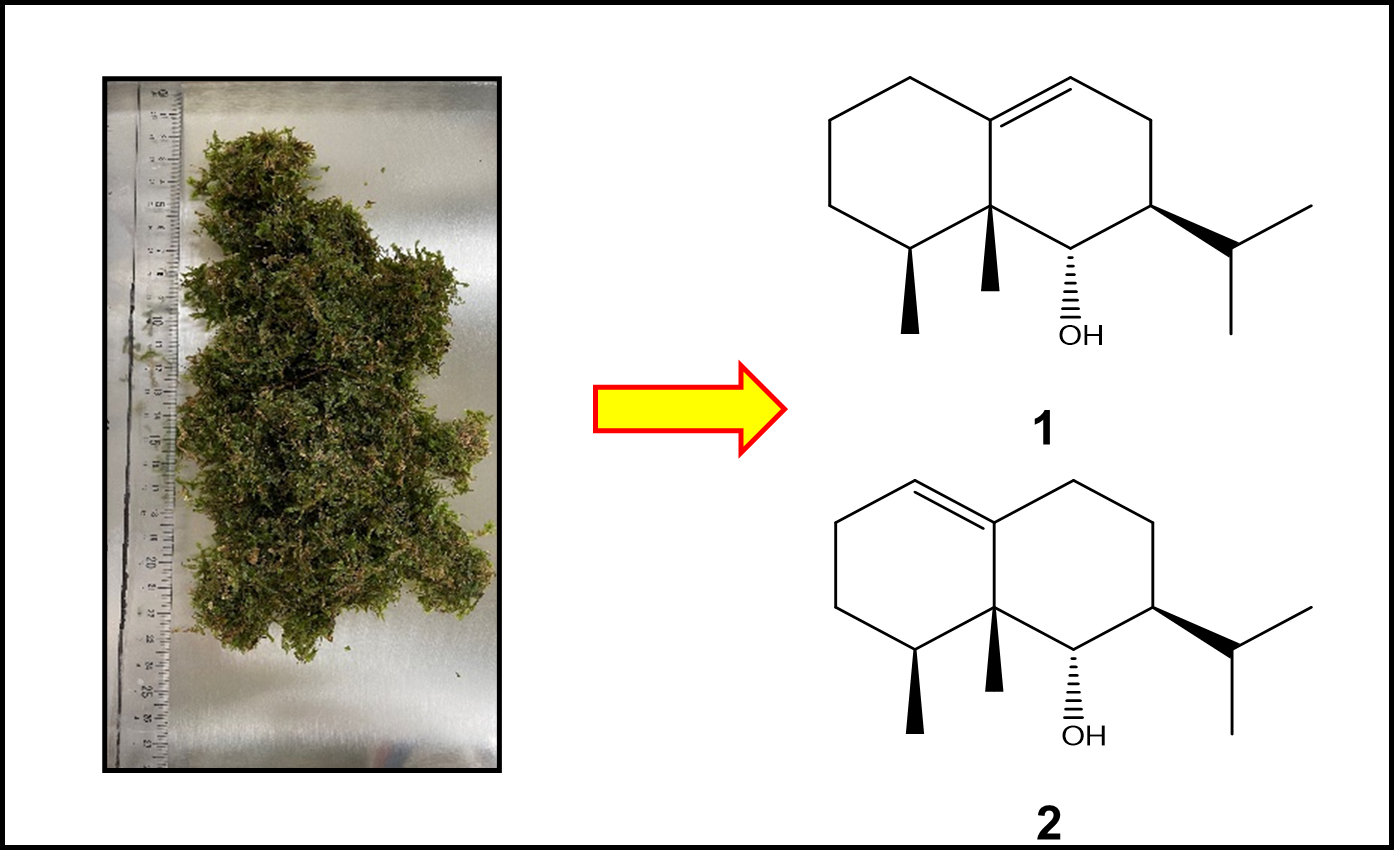
"Two New Eremophilane-Type Sesquiterpenoids from Japanese Liverwort Bazzania japonica" R. Fukada, J. Kawano, T. Tsuruta, T. Nonaka, K. Sato, S. Miyazawa, S. Ishigami, T. Ishii, K. Nishikawa, Y. Asakawa, and T. Kamada Chem. Biodivers. 20, e202300131 (2023).
ABSTRACT: Two new eremophilane-type sesquiterpenoids, fusumaols A (1) and B (2), were isolated from the stem-leafy liverwort, Bazzania japonica collected in Mori-Machi, Shizuoka, Japan. Their structures were established using extensive spectroscopic (IR, MS, and 2D NMR) data, and the absolute configuration of 1 was determined by the modified Mosher's method. This is the first time eremophilanes have been discovered in the liverwort genus Bazzania. Compounds 1 and 2 were evaluated for their repellent activity against the adult population of the rice weevil Sitophilus zeamais using the modified filter paper impregnation method. Both sesquiterpenoids showed moderate repellent activities.

"ヨウ化サマリウムによるバービアー型環化反応を鍵工程とするトキシコデナン類の不斉全合成: 糖尿病性腎症の低分子創薬シード分子の探索 ( Asymmetric Total Synthesis of Toxicodenanes by the Samarium Iodide-Induced Barbier-Type Cyclization Reaction as a Key Step: Seeking of Seed Compounds for the Small Molecule Drug Discovery of Diabetic Nephropathy )" K. Nishikawa and Y. Morimoto 月刊ファインケミカル 52, 36-44 (2023).

"Divergent Synthesis of Nerolidol-Type Sesquiterpenoids Produced by Soil Bacterium from an Identical Starting Material via Diepoxide Precursors: Stereochemical Revision and Absolute Configuration of a THF Natural Product" T. Teranishi, K. Nishikawa, A. Matsuura, M. Kumagai, and Y. Morimoto Chem. Lett. 51, 1062-1066 (2022).
ABSTRACT: A divergent asymmetric synthesis of two nerolidol-type sesquiterpenoids with a five- or a six-membered ether ring was established from an identical commercially available trans,trans-farnesyl acetate through the cyclization of diepoxide precursors under simple acidic or neutral conditions, respectively. In addition, the relative configuration of a nerolidol-type sesquiterpenoid with a tetrahydrofuran ring was revised. Its absolute configuration was determined by its asymmetric synthesis and a modified Mosher’s analysis. Furthermore, the cytotoxicity and nitric oxide production inhibitory activity of the synthesized compounds were assessed.
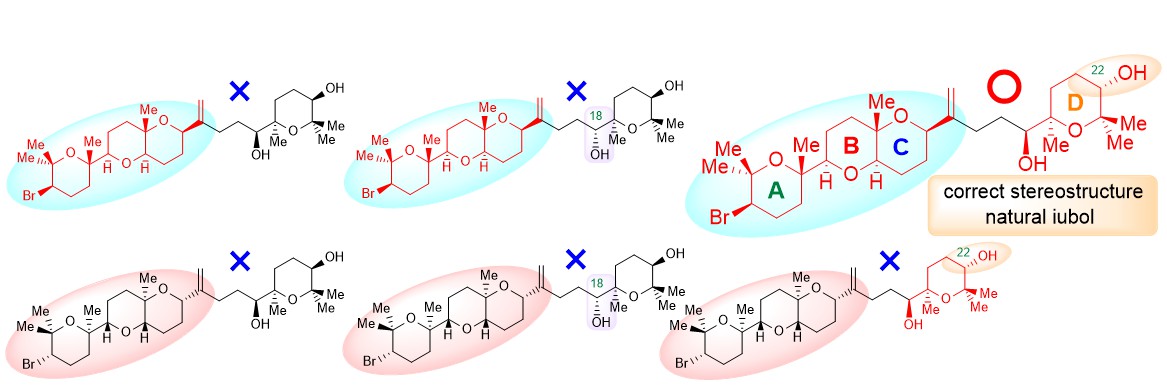
"Total Synthesis, Revised Structure, and Cytotoxic Activities of Iubol" K. Nishikawa, N. Taki, K. Nishikibe, M. Kumagai, and Y. Morimoto Chem. Lett. 51, 1000-1003 (2022).
ABSTRACT: To unambiguously establish the stereostructure of the marine cytotoxic bromotriterpenoid iubol, a member of the thyrsiferol family, asymmetric chemical synthesis has been carried out. The synthesis features a one-pot process for the tetrahydropyranyl D ring construction through a stoichiometric Sharpless asymmetric epoxidation of allylic alcohols followed by titanium chelation-assisted 6-exo oxacyclization. In this paper, we report the asymmetric total synthesis of iubol, revision of the proposed structure to the C22-epimer, and preliminary cytotoxic activities of synthetic compounds against some tumor cells.

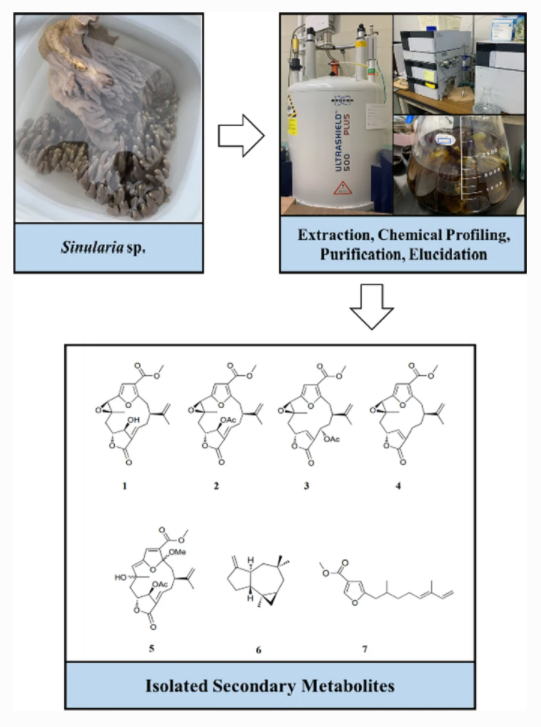
"Furanocembranoid from the Okinawan Soft Coral Sinularia sp." M. Nagasaka, K. Tani, K. Nishikawa, R. Kinjo, and T. Ishii Nat. Prod. Bioprospect. 12, Article number 7 (2022) [ Open Access ].
ABSTRACT: One new furanocembranoid diterpene, 11-hydroxy-Δ12(13)-pukalide (1), along with six known secondary metabolites, 11-acetoxy-Δ12(13)-pukalide (2), 13α-acetoxypukalide (3), pukalide (4), 3α-methoxyfuranocembranoid (5), Δ9(15)-africanene (6), and methyl (5′E)-5-(2′,6′-dimethylocta-5′,7′-dienyl)furan-3-carboxylate (7) were isolated from the Okinawan soft coral Sinularia sp. Their chemical structures were elucidated based on spectroscopic analysis (FTIR, NMR, and HRESIMS), and the relative stereochemistry of 1 was determined by NOESY experiments and acetylation, which yielded derivative 2. In addition, compounds 1 and 7 exhibited toxicity in the brine shrimp lethality test.
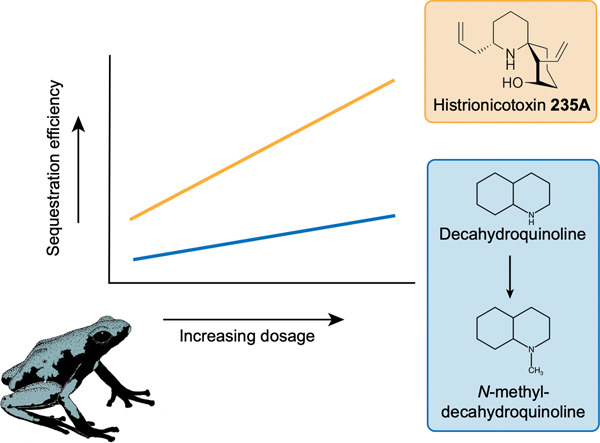
"Dose-Dependent Alkaloid Sequestration and N-Methylation of Decahydroquinoline in Poison Frogs" A. M. Jeckel, S. K. Bolton, K. R. Waters, M. M. Antoniazzi, C. Jared, K. Matsumura, K. Nishikawa, Y. Morimoto, T. Grant, and R. A. Saporito J. Exp. Zool. A Ecol. Integr. Physiol. 337, 537-546 (2022).
ABSTRCT: Sequestration of chemical defenses from dietary sources is dependent on the availability of compounds in the environment and the mechanism of sequestration. Previous experiments have shown that sequestration efficiency varies among alkaloids in poison frogs, but little is known about the underlying mechanism. The aim of this study was to quantify the extent to which alkaloid sequestration and modification are dependent on alkaloid availability and/or sequestration mechanism. To do this, we administered different doses of histrionicotoxin (HTX) 235A and decahydroquinoline (DHQ) to captive-bred Adelphobates galactonotus and measured alkaloid quantity in muscle, kidney, liver, and feces. HTX 235A and DHQ were detected in all organs, whereas only DHQ was present in trace amounts in feces. For both liver and skin, the quantity of alkaloid accumulated increased at higher doses for both alkaloids. Accumulation efficiency in the skin increased at higher doses for HTX 235A but remained constant for DHQ. In contrast, the efficiency of HTX 235A accumulation in the liver was inversely related to dose and a similar, albeit statistically nonsignificant, pattern was observed for DHQ. We identified and quantified the N-methylation of DHQ in A. galactonotus, which represents a previously unknown example of alkaloid modification in poison frogs. Our study suggests that variation in alkaloid composition among individuals and species can result from differences in sequestration efficiency related to the type and amount of alkaloids available in the environment.
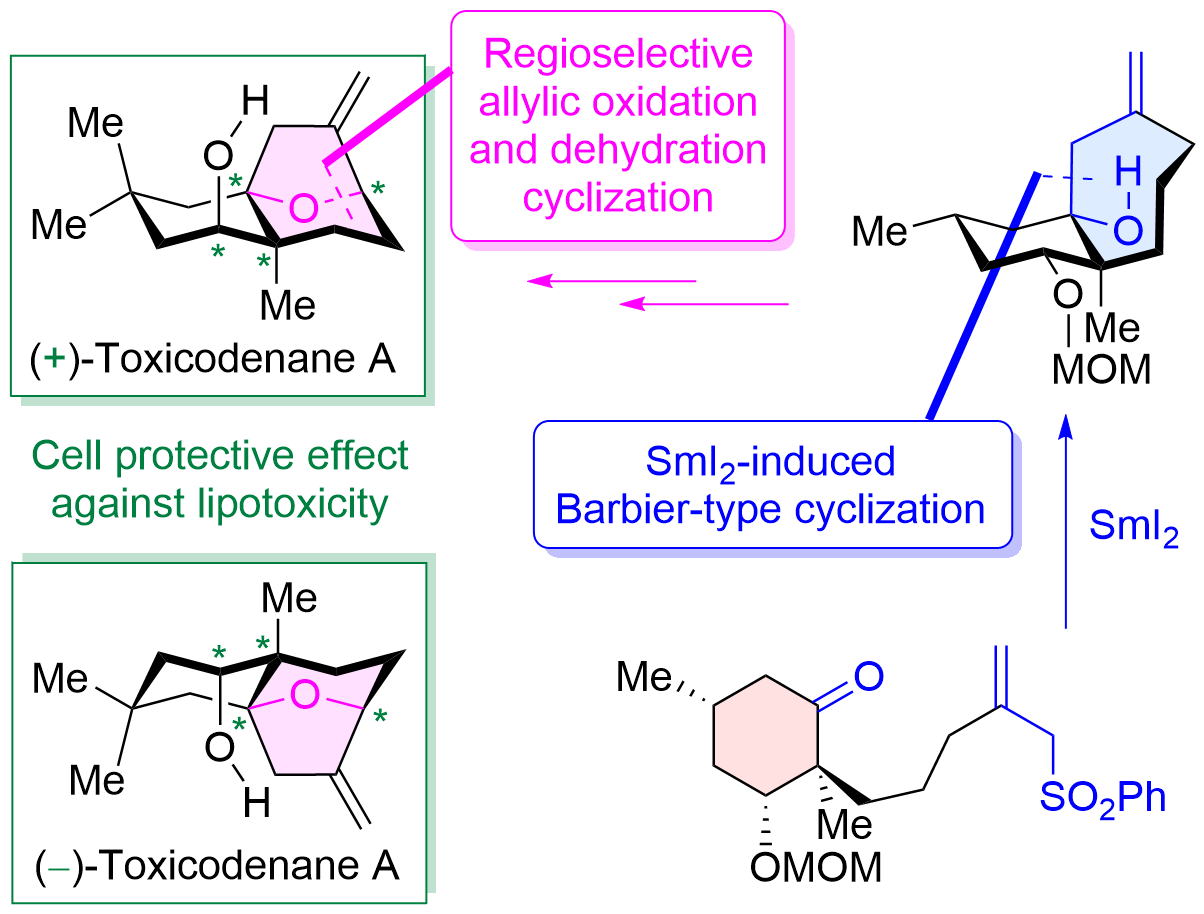
"Asymmetric Total Synthesis of Toxicodenane A by Samarium-Iodide-Induced Barbier-Type Cyclization and Its Cell-Protective Effect against Lipotoxicity" K. Nishikawa, K. Kikuta, T. Tsuruta, H. Nakatsukasa, S. Sugahara, S. Kume, and Y. Morimoto Org. Lett. 24, 531-535 (2022).
Selected as Supplementary Cover Art.
Highlighted in Synfacts 18, 0441 (2022).
ABSTRACT: The asymmetric total synthesis of toxicodenane A, a sesquiterpenoid expected to be promising for diabetic nephropathy, was achieved. In the synthesis, a samarium iodide (SmI2)-induced Barbier-type cyclization and a regio- and stereoselective allylic oxidation followed by a dehydration cyclization were employed as key steps. Furthermore, the first asymmetric syntheses of both enantiomers were accomplished using the previously mentioned synthetic strategy. Finally, the synthetic compounds significantly inhibited lipotoxicity-mediated inflammatory and fibrotic responses in mouse renal proximal tubular cells.

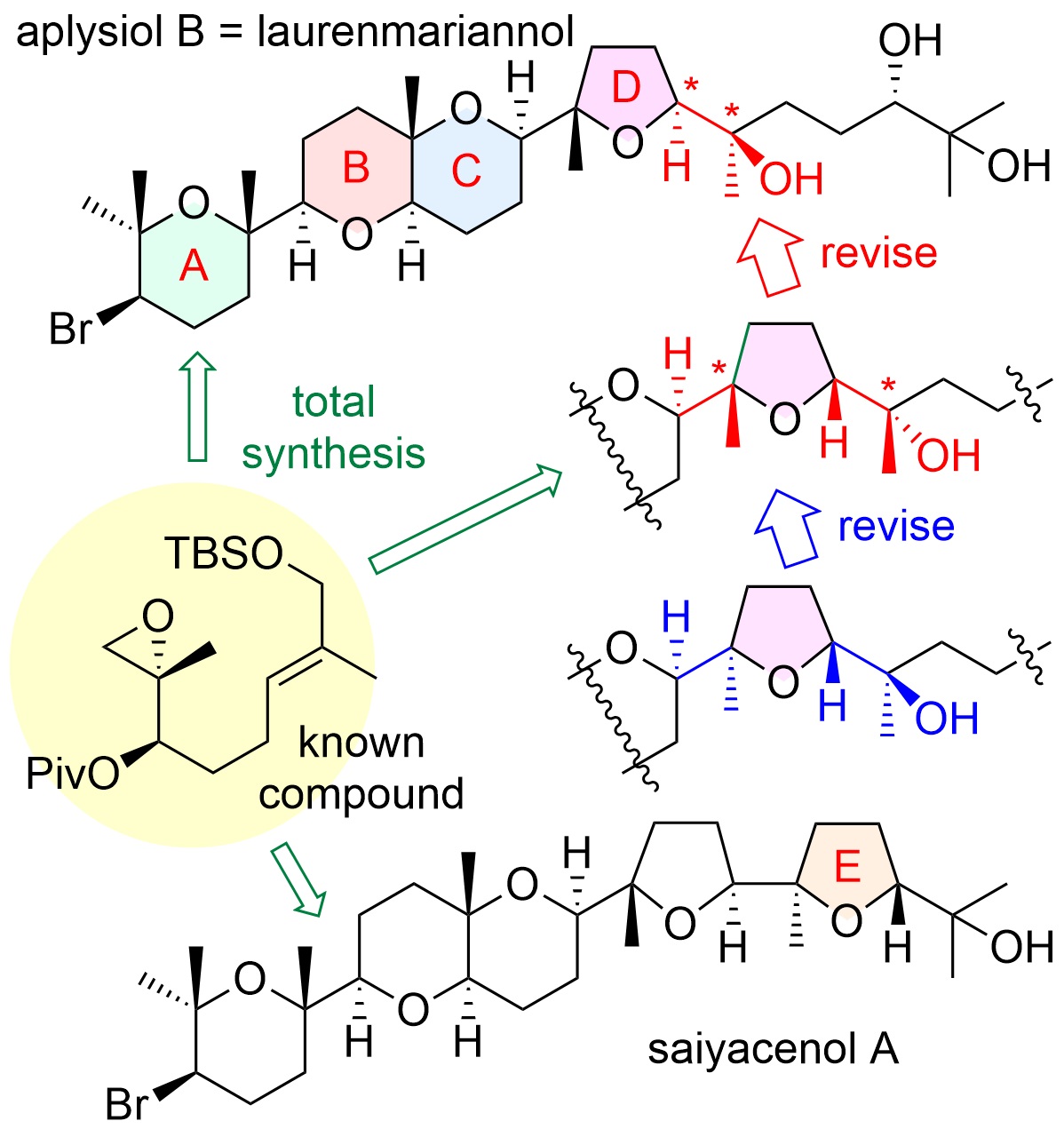
"Asymmetric Total Syntheses, Stereostructures, and Cytotoxicities
of Marine Bromotriterpenoids Aplysiol B (Laurenmariannol) and Saiyacenol
A" K. Nishikibe, K. Nishikawa, M. Kumagai, M. Doe, and Y. Morimoto
Chem. Asian J. 17, e202101137 (2022) [ Open Access ].
Selected as Front Cover.
ABSTRACT: Total assignments of stereostructures of marine cytotoxic bromotriterpenoids,
aplysiol B (laurenmariannol) and saiyacenol A, were accomplished through
their asymmetric chemical syntheses to elucidate their ambiguous stereostructures
and whether or not other members showing the opposite chirality for the
ABC ring system exist. Moreover, their preliminary growth inhibitory activities
against some tumor cells were also evaluated.

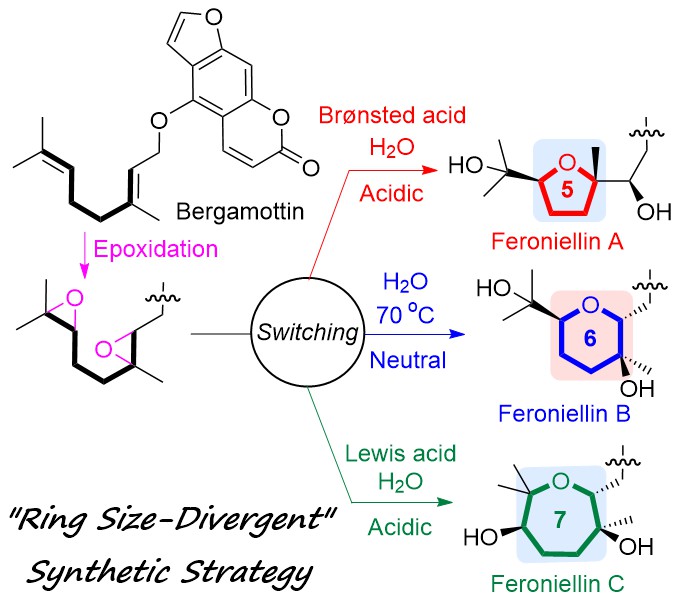
"Establishing a “Ring Size-Divergent” Synthetic Strategy: Synthesis,
Structural Revision, and Absolute Configuration of Feroniellins" K.
Nishikawa, T. Niwa, K. Nishikibe, M. Kumagai, and Y. Morimoto Chem. Eur. J. 27, 11045–11049 (2021).
ABSTRACT: A “ring-size-divergent” strategy enabled us to divergently synthesize
the five-, six-, and seven-membered ether rings of feroniellin analogs
from diepoxides under simple acidic or neutral conditions in only two steps
from bergamottin. Additionally, the proposed structures of feroniellins
A and B were revised, and the absolute configurations of all feroniellins
were determined from their asymmetric synthesis.
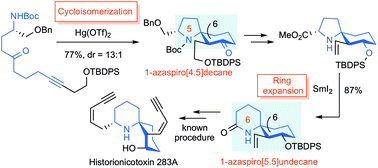
"One‐Step Synthesis of the 1‐Azaspiro[5.5]undecane Skeleton Characteristic
of Histrionicotoxin Alkaloids from Linear Substrates via Hg(OTf)2‐Catalyzed Cycloisomerization" K. Matsumura, K. Nishikawa, H. Yoshida,
T. Niwa, Y. Fushii, M. Doe, and Y. Morimoto Chem. Asian J. 16, 1882–1886 (2021).
ABSTRACT: A one-step synthesis of the 1-azaspiro[5.5]undecane skeleton in histrionicotoxin
alkaloids from a linear substrate was realized utilizing a Hg(OTf)2-catalyzed cycloisomerization reaction. Histrionicotoxin alkaloids as potential
target drugs were successfully synthesized via our developed cyclization.
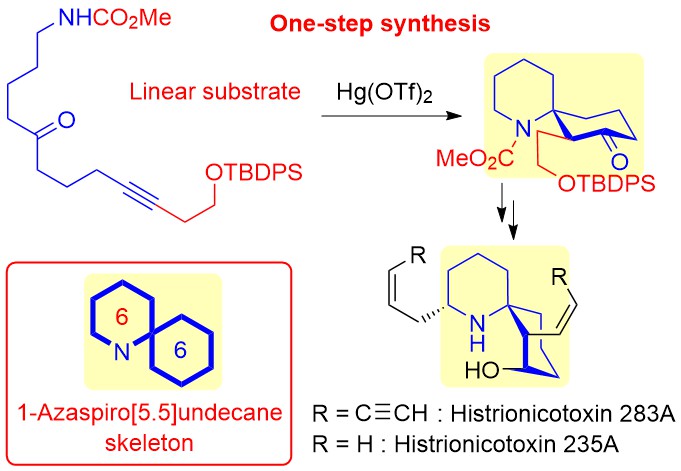
"Tetrodotoxin Framework Construction from Linear Substrates Utilizing
a Hg(OTf)2-Catalyzed Cycloisomerization Reaction: Synthesis of the Unnatural Analogue 11-nor-6,7,8-Trideoxytetrodotoxin" K. Nishikawa, T. Noguchi, S. Kikuchi, T. Maruyama, Y. Araki, M. Yotsu-Yamashita, and Y. Morimoto Org. Lett. 23, 1703–1708 (2021).
ABSTRACT: In this contribution, we propose a new synthetic approach to tetrodotoxin
(TTX), one of the most famous marine toxins that, after first preparing
a functionalized linear substrate, forms a cyclohexane core from the substrate
utilizing our mercuric triflate (Hg(OTf)2)-catalyzed cycloisomerization reaction. The concept was applied to the synthesis of 11-nor-6,7,8-trideoxyTTX and 11-nor-4,9-anhydro-6,7,8-trideoxyTTX, which are unnatural TTX analogues, demonstrating
the validity of our new approach.
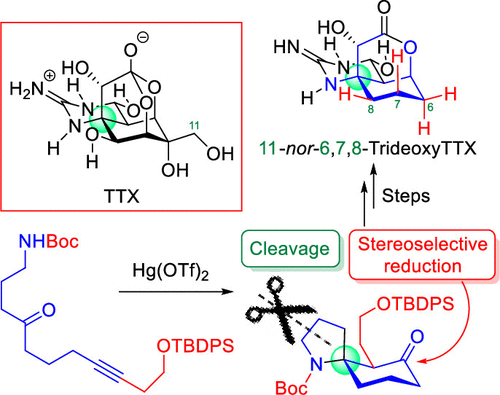
"Natural Product Synthesis Strategy Based on the Concept of Directly
Constructing the Ring Skeletons from Linear Substrates" K. Nishikawa,
M. Kumagai, K. Matsumura, K. Nishikibe, and Y. Morimoto J. Synth. Org. Chem., Jpn. 79, 197-209 (2021).
ABSTRACT: Our concept of natural product synthesis is to directly construct the
ring skeletons, frequently occurring in natural products with strong biological
activities, from easily accessible linear substrates, and our group has
established practical methods for synthesizing useful natural products
along our synthetic strategy. First, we developed a cycloisomerization
reaction to directly synthesize nitrogen-containing spirocycles from linear
substrates, accompanied with construction of a stereogenic tetrasubstituted
spiro-carbon. The reaction enabled us to achieve the efficient total synthesis
of lepadiformines, revealing a phenomenon of their enantiodivergence. The
synthesis of histrionicotoxins, frog poisons, was also accomplished through
our original cyclization reaction. In addition, we found the critical switching
of cyclization modes of polyepoxides in acidic aqueous media and neutral
water, and the novel cascade cyclization was applied to the synthesis of
natural products.
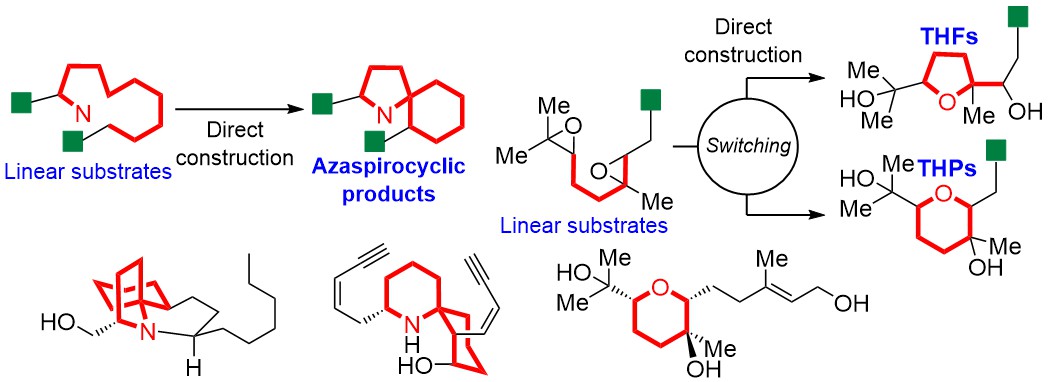
"4β-Hydroxywithanolide E and Withanolide E from Physalis peruviana L. Inhibit Adipocyte Differentiation of 3T3-L1 Cells through Modulation
of Mitotic Clonal Expansion" M. Kumagai, I. Yoshida, T. Mishima, M.
Ide, K. Fujita, M. Doe, K. Nishikawa, and Y. Morimoto J. Nat. Med. 75, 232–239 (2021).
ABSTRACT: Obesity is a risk factor for many diseases, including type 2 diabetes and cardiovascular disease, and is related to the rising morbidity and mortality. Discovery of agents targeting adipogenesis, especially from natural sources, is important for the treatment of obesity. Here, we aimed to identify anti-adipogenic substances in methanol extracts of Physalis peruviana and to investigate their effect, along with underlying mechanisms. Activity-guided
fractionation of the extract revealed 4β-hydroxywithanolide E (HWE) and
withanolide E (WE) as the adipogenesis inhibitors. Both compounds suppressed
mRNA expression of central adipogenic transcription factors, peroxisome
proliferator-activated receptor γ, and CCAAT/enhancer-binding protein α
in the early stage of adipocyte differentiation. The inhibitory action
of these two withanolides on adipogenesis was largely limited to this stage.
The proliferation of preadipocytes was markedly suppressed by treatment
with HWE and WE for 24 and 48 h in the differentiation medium, and cell-cycle
arrest in the G0/G1 phase was observed. Therefore, our results suggested
that withanolides from P. peruviana to be novel anti-adipogenic compounds that modulate mitotic clonal expansion.
”Use of Whole‐Body Cryosectioning and Desorption Electrospray Ionization
Mass Spectrometry Imaging to Visualize Alkaloid Distribution in Poison
Frogs" A. M. Jeckel, K. Matsumura, K. Nishikawa, Y. Morimoto, R. A.
Saporito, T. Grant, and D. R. Ifa J. Mass Spectrom. 55, e4520 (2020).
ABSTRACT: Ambient mass spectrometry is useful for analyzing compounds that would
be affected by other chemical procedures. Poison frogs are known to sequester
alkaloids from their diet, but the sequestration pathway is unknown. Here,
we describe methods for whole-body cryosectioning of frogs and use desorption
electrospray ionization mass spectrometry imaging (DESI-MSI) to map the
orally administered alkaloid histrionicotoxin 235A in a whole-body section
of the poison frog Dendrobates tinctorius. Our results show that whole-body
cryosectioning coupled with histochemical staining and DESI-MSI is an effective
technique to visualize alkaloid distribution and help elucidate the mechanisms
involved in alkaloid sequestration in poison frogs.
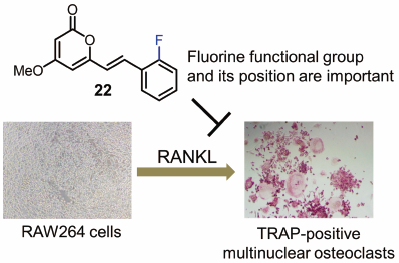
"Fluorinated Kavalactone Inhibited RANKL-Induced Osteoclast Differentiation
of RAW264 Cells" M. Kumagai, K. Nishikawa, T. Mishima, I. Yoshida,
M. Ide, A. Watanabe, K. Fujita, and Y. Morimoto Biol. Pharm. Bull. 43, 898–903 (2020) [ Open Access ].
ABSTRACT: Bone loss and bone-related disease are associated with the deregulation
of osteoclast function, and therefore agents that affect osteoclastogenesis
have attracted attention. The purpose of the present study was to discover
modified kavalactone analogs as potential anti-osteoclastogenic agents.
We assessed the effect of 26 analogs on osteoclast differentiation in vitro. The most potent compound, (E)-6-(2-fluorostyryl)-4-methoxy-2H-pyran-2-one (22), suppressed receptor activator of nuclear factor-κB ligand (RANKL)-induced
osteoclastogenic differentiation of RAW264 cells with IC50 values of 4.3 µM. A partial structure–activity relationship study revealed
the importance of fluorine and its position within the 5,6-dehydrokawain
skeleton. The results of a pit formation assay suggested that compound
22 prevents osteoclastic bone resorption by inhibiting osteoclastogenesis. Moreover, compound 22 downregulated mRNA expression levels of RANKL-induced nuclear factor of
activated T cells c1 (NFATc1) and osteoclastogenesis-related genes. These
results suggest that (E)-6-(2-fluorostyryl)-4-methoxy-2H-pyran-2-one scaffold could lead to the identification of new anti-resorptive
agents.
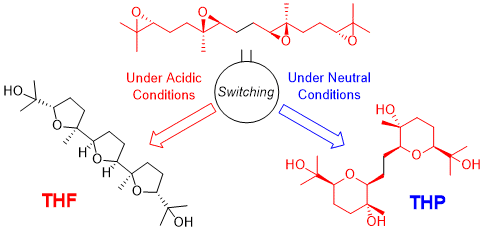
"Critical Switching of Cyclization Modes of Polyepoxides in Acidic
Aqueous Media and Neutral Water: Synthesis and Revised Structure of a Nerolidol‐Type
Sesquiterpenoid" K. Nishikawa, K. Morita, S. Hashimoto, A. Hoshino,
T. Ikeuchi, M. Kumagai, and Y. Morimoto Angew. Chem. Int. Ed. 58, 10168–10172 (2019);
Angew. Chem. 131, 10274–10278 (2019).
Selected as Front Cover.
ABSTRACT: Biomimetic epoxide-opening cascades of polyepoxides enable the efficient
and rapid construction of polyether frameworks. Herein, we show that the
epoxide-opening cascade cyclization that affords tetrahydrofuran products
in acidic aqueous media produces tetrahydropyran (THP) in neutral water.
THP formation proceeded by simply heating polyepoxides in neutral water
and followed a different cyclization mode from those observed so far. The
novel cascade cyclization in H2O was applied to the synthesis of a new nerolidol-type sesquiterpenoid,
resulting in revision of the proposed structure and determination of the
absolute configuration.

“Formal Total Synthesis of Histrionicotoxin Alkaloids via Hg(OTf)2-catalyzed Cycloisomerization and SmI2-induced Ring Expansion” K. Matsumura, K. Nishikawa, H. Yoshida, M. Doe,
and Y. Morimoto RSC Adv. 8, 11296-11303 (2018) [ Open Access ].
ABSTRACT: The efficient formal total synthesis of histrionicotoxin alkaloids was
achieved. In this process, two key reactions were used to construct a core
1-azaspiro[5.5]undecane framework common to histrionicotoxins: a mercuric
triflate (Hg(OTf)2)-catalyzed cycloisomerization of a linear substrate, which was developed
in our laboratory, and a samarium iodide (SmI2)-mediated ring expansion.
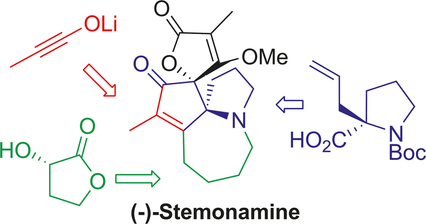
"Asymmetric Total Synthesis of (−)-Stemonamine and Its Stereochemical Stability" S. Fujita, K. Nishikawa, T. Iwata, T. Tomiyama, H. Ikenaga, K. Matsumoto, and M. Shindo Chem. Eur. J. 24, 1539-1543 (2018).
ABSTRACT: The first total synthesis of nonracemic (−)-stemonamine is achieved in
17 steps from known compounds. Key reactions include intramolecular acylation
and ynolate-initiated tandem [2+2] cycloaddition-Dieckmann condensation.
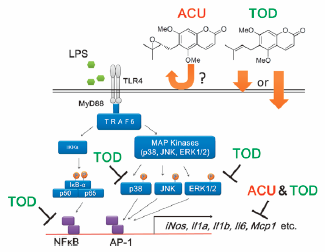
"Evaluation of Aculeatin and Toddaculin Isolated from Toddalia asiatica as Anti-inflammatory Agents in LPS-Stimulated RAW264 Macrophages"
M. Kumagai, A. Watanabe, I. Yoshida, T. Mishima, M. Nakamura, K. Nishikawa,
and Y. Morimoto Biol. Pharm. Bull. 41, 132-137 (2018) [ Open Access ].
ABSTRCT: Anti-inflammatory activity of aculeatin and toddaculin, which are coumarins
with a similar structure isolated from Toddalia asiatica (L.) LAM., was evaluated using lipopolysaccharide (LPS)-stimulated RAW264 mouse macrophage cells. Both aculeatin and toddaculin significantly inhibited mRNA expression of inflammatory mediators and nitric oxide production. Furthermore, Toddaculin suppressed LPS-induced phosphorylation of p38 and extracellular signal-regulated kinase (ERK)1/2 and inhibited LPS-induced activation of nuclear factor-kappaB (NF-κB). However, aculeatin did not exhibit such effects, suggesting that aculeatin and toddaculin suppress LPS-induced inflammation of RAW264 cells via different mechanisms. The cellular uptake of these compounds was also evaluated. Toddaculin was detected in RAW264 cells after 4 and 24 h. However, aculeatin levels were not observed in RAW264 cells at all incubation intervals. These results indicate that de-epoxidation of a prenyl group can increase hydrophobicity of molecule and is thought to accelerate cellular uptake and/or interactions with the phospholipid bilayers of cell membranes.
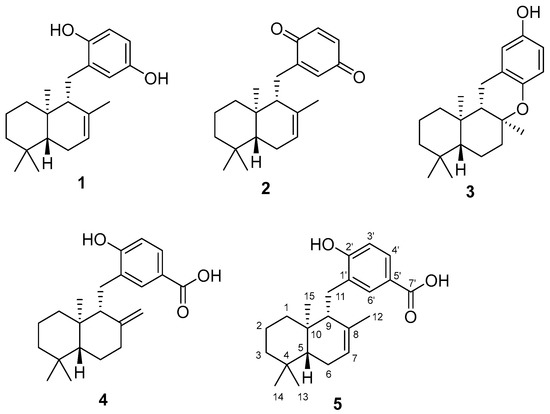
"Antioxidants from the Brown Alga Dictyopteris undulata" M. Kumagai, K. Nishikawa, H. Matsuura, T. Umezawa, F. Matsuda, and
T. Okino Molecules 23, 1214 (2018) [ Open Access ].
ABSTRACT: An investigation of anti-oxidative compounds from the brown alga Dictyopteris undulata has led to the isolation and identification of isozonarol, isozonarone, chromazonarol, zonaroic acid and isozonaroic acid. Their structures were identified by comparison of MS and NMR spectra. Full NMR assignment and absolute configuration of isozonaroic acid are described. Isozonarol showed the most potent 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging activity among the compounds isolated.
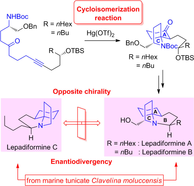
"Total Syntheses of Lepadiformine Marine Alkaloids with Enantiodivergency,
Utilizing Hg(OTf)₂-Catalyzed Cycloisomerization Reaction and Their Cytotoxic
Activities" K. Nishikawa, K. Yamauchi, S. Kikuchi, S. Ezaki, T. Koyama, H. Nokubo, K. Matsumura, T. Kodama, M. Kumagai, and Y. Morimoto Chem. Eur. J. 23, 9535-9545 (2017).
Selected as Back Cover.
ABSTRACT: A phenomenon of enantiodivergence was found in lepadiformine alkaloids isolated from a single species marine tunicate Clavelina moluccensis through their syntheses. The enantioselective total syntheses have been achieved by a key mercury(II) triflate-catalyzed cycloisomerization reaction developed in our laboratory (see scheme), and cytotoxic activities of synthesized compounds were also evaluated.

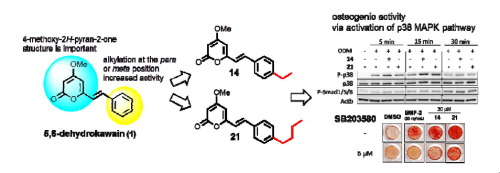
"Synthesis of Novel 5,6-Dehydrokawain Analogs as Osteogenic Inducers and Their Action Mechanisms" M. Kumagaia, K. Nishikawa, T. Mishima, I. Yoshida, M. Ide, K. Koizumi, M. Nakamura, and Y. Morimoto Bioorg. Med. Chem. Lett. 27, 2401–2406 (2017).
ABSTRACT: An imbalance between bone resorption by osteoclasts and bone formation
by osteoblasts can cause bone loss and bone-related disease. In a previous
search for natural products that increase osteogenic activity, we found
that 5,6-dehydrokawain (1) from Alpinia zerumbet promotes osteoblastogenesis. In this study, we synthesized and evaluated
series of 5,6-dehydrokawain analogs. Our structure-activity relationships
revealed that alkylation of para or meta position of aromatic ring of 1
promote osteogenic activity. Among the potential analogs we synthesized,
(E)-6-(4-Ethylstyryl)-4-methoxy-2H-pyran-2-one (14) and (E)-6-(4-Butylstyryl)-4-methoxy-2H-pyran-2-one (21) both significantly up-regulated Runx2 and Osterix mRNA expression at
10 µM. These osteogenic activities could be mediated by bone morphogenetic
protein (BMP) and activation of p38 MAPK signaling pathways. Compounds
14 and 21 also inhibited RANKL-induced osteoclast differentiation of RAW264 cells.
These results indicated that novel 5,6-dehydrokawain analogs not only increase
osteogenic activity but also inhibit osteoclast differentiation, and could
be potential lead compounds for the development of anti-osteoporosis agents.
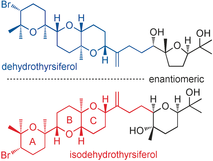
"Total Synthesis of the Cytotoxic Marine Triterpenoid Isodehydrothyrsiferol
Reveals Partial Enantiodivergency in the Thyrsiferol Family of Natural
Products" A. Hoshino, H. Nakai, M. Morino, K. Nishikawa, T. Kodama,
K. Nishikibe, and Y. Morimoto Angew. Chem. Int. Ed. 56, 3064-3068 (2017);
Angew. Chem. 129, 3110–3114 (2017).
ABSTRACT: Enantioselective total synthesis of the cytotoxic marine triterpenoid
isodehydrothyrsiferol revealed partial enantiodivergence in that the ABC
ring system is enantiomeric to that of the other members of this natural
product family. Furthermore, this unprecedented partial enantiodivergence
is observed between dehydrothyrsiferol and isodehydrothyrsiferol, which
originate from a single species, the red alga Laurencia viridis.
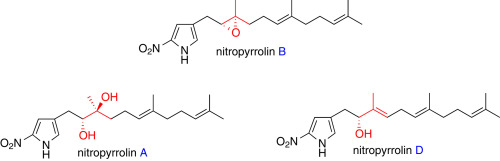
"Total Synthesis of Nitropyrrolins A, B, and D" H. Mitani, T.
Matsuo, T. Kodama, K. Nishikawa, Y. Tachi, and Y. Morimoto Tetrahedron 72, 7179–7184 (2016).
ABSTRACT: The chemical synthetic method of cytotoxic nitropyrrolins A (1), B (2), and D (4), 2-nitropyrrole terpenoids rarely occurring in nature, has been developed
for further biological studies. After the synthesis of nitropyrrolin B
has first been achieved, nitropyrrolin B was transformed into nitropyrrolins
A and D in two steps and one step, respectively. The regio- and stereoselective
epoxide-cleavage reaction was highlighted in the direct conversion of nitropyrrolin
B to D.
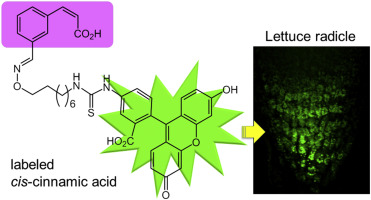
"Synthesis of Fluorescent Molecular Probes Based on cis-Cinnamic Acid and Molecular Imaging of Lettuce Roots" H. Fukud, K.
Nishikawa, Y. Fukunaga, K. Okuda, K. Kodama, K. Matsumoto, A. Kano, and
M. Shindo Tetrahedron 72, 6492-6498 (2016).
Selected as Front Cover.
ABSTRACT: We synthesized azo dye- and fluorescence-labeled cis-cinnamic acid analogues possessing inhibitory activity against lettuce
root growth and a trans-isomer without bioactivity as a control probe. The radicles incubated with the azo dye-labeled analogue were stained red, with their tips especially deeply dyed. The fluorescent images of the radicles incubated with each of these molecular probes depicted that the root cap was fluorescence-stained. However, images of the control radicles prepared by staining with the trans-isomer fluorescent probe did not show emission at the root cap. These contrasts suggest specific localization of the cis-cinnamate analogue at the columella cells.

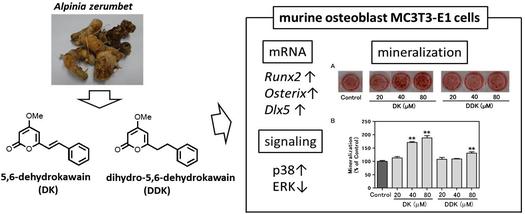
"5,6-Dehydrokawain from Alpinia zerumbet Promotes Osteoblastic MC3T3-E1 Cell Differentiation" M. Kumagai, T. Mishima, A. Watanabe, T. Harada, I. Yoshida, K. Fujita,
M. Watai, S. Tawata, K. Nishikawa, and Y. Morimoto Biosci. Biotech. Biochem. 80, 1425-1432 (2016) [ Open Access ].
ABSTRACT: Bone homeostasis is maintained by balancing bone formation and bone resorption, but an imbalance between them is associated with various bone-related diseases such as osteoporosis and rheumatoid arthritis. We found that 5,6-dehydrokawain (DK) and dihydro-5,6-dehydrokawain (DDK), which were isolated aspromising compounds from Alpinia zerumbet rhizomes, promote differentiation of osteoblastic MC3T3-E1 cells. DK and DDK increased the alkaline phosphatase activity and matrix mineralization of MC3T3-E1 cells. DK exerts larger effects than DDK. The gene expression of runt-related transcription factor 2 and osterix, which are essential transcription factors in the early period of osteoblast differentiation, was significantly increased by DK treatment. The mRNA level of distal-less homeobox 5 was also enhanced by DK treatment, and DK activated the p38 mitogen-activated protein kinase pathway. Therefore, DK may have clinical potential for preventing osteoporosis, and could be considered as a potential anabolic therapeutic agent. 5,6-Dehydrokawain (DK) and dihydro-5,6-dehydrokawain (DDK) from Alpinia zerumbet rhizomes, promote differentiation of osteoblastic MC3T3-E1 cells.
"Total Synthesis of Natural Antifouling Products" T. Umezawa,
K. Nishikawa, T. Okino, and F. MatsudaJ. Synth. Org. Chem., Jpn. 74, 689-699 (2016).
ABSTRACT: Biofouling is adverse growth of marine organisms on manmade submersible structures such as ships’ hulls and cause significant economic and environmental problems. As a fouling inhibitor, tributyltin (TBT) has been widely used for controlling the sessile organisms since the early 1960s. Unfortunately, serious pollution of the marine environment due to the deleterious effect of TBT prompted the International Maritime Organization (IMO) to call in 2008 for a ban on the use of tributyltin (TBT) on ships. Since marine invertebrates prevent settlement of other benthic marine organisms through the use of natural substances with antifouling properties without causing serious environmental problems, natural antifouling products with good antifouling properties but without biocidal properties have attracted considerable attention. Among these, 10-isocyano-4-cadinene and omaezallene show promise as lead compounds for the development of new environmentally friendly antifouling agents due to its potent antifouling activity against the cypris larvae of the barnacle Amphibalanus amphitrite and low toxicity. 10-Isocyano-4-cadinene, an isocyanosesquiterpene, was
isolated from nudibranchs of the family Phyllidiidae. On the other hand, omaezallene is a bromoallene-containing C15-acetogenin
isolated from the red alga Laurencia sp. Herein, we wish to describe our research on the isolations, structure elucidations, total syntheses, and evaluation of the antifouling activities of the natural products and their derivatives. The absolute configurations of the natural products were unambiguously established through our asymmetric total syntheses.
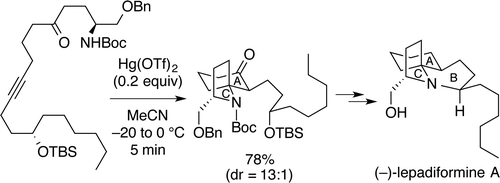
"Total Synthesis of (−)-Lepadiformine A Utilizing Hg(OTf)2-Catalyzed Cycloisomerization Reaction" K. Nishikawa, S. Kikuchi,
S. Ezaki, T. Koyama, H. Nokubo, T. Kodama, Y. Tachi, and Y. Morimoto Org. Lett. 17, 5772-5775 (2015);
Highlighted in Synfacts 2, 116 (2016).
ABSTRACT: A cytotoxic marine alkaloid (−)-lepadiformine A (1) possesses a unique structure characterized by the trans-1-azadecalin AB ring system fused with the AC spiro-cyclic ring. In this research, we found that a cycloisomerization reaction from amino ynone 2 to a 1-azaspiro[4.5]decane skeleton 3, corresponding to the AC ring system of 1, is promoted by Hg(OTf)2. Thus, we have accomplished the efficient total synthesis of (−)-lepadiformine
A in 28% overall yield by featuring the novel Hg(OTf)2-catalyzed cycloisomerization.
"Total Synthesis and Complete Stereochemical Assignment of Heronapyrroles
A and B" T. Matsuo, S. Hashimoto, K. Nishikawa, T. Kodama, S. Kikuchi,
Y. Tachi, Y. Morimoto Tetrahedron Lett. 56, 5345-5348 (2015).
ABSTRCT: Heronapyrroles A and B with promising and selective antibacterial activity
but no cytotoxicity against mammalian cell lines are among members of rare
and unique 4-farnesylated-2-nitropyrrole natural products. The absolute
configurations at C7 and C15 have been proposed to be 7S and 15R by the modified Mosher method. In this Letter, we have achieved the total synthesis of natural (+)-heronapyrroles A (1) and B (2) and report that the correct absolute configurations are 7R, 8S, and 15S as shown in the structural formulas 1 and 2.

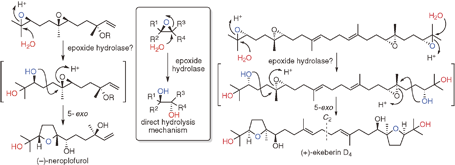
"Biomimetic Total Synthesis of (-)-Neroplofurol and (+)-Ekeberin D4
Triggered by Hydrolysis of Terminal Epoxides" T. Kodama, S. Aoki,
T. Matsuo, Y. Tachi, K. Nishikawa, and Y. Morimoto Chem. Lett. 43, 1662-1664 (2014).
ABSTRACT: To accumulate the chemical basis of epoxide-opening cascade biogenesis,
chemical syntheses of sesqui- and triterpenoids were performed. The biomimetic
total syntheses of (−)-neroplofurol (1) and (+)-ekeberin D4 (2) were accomplished by protic acid-catalyzed hydrolysis of the terminal
epoxide from nerolidol diepoxide 3 and squalene tetraepoxide 4 through single and double 5-exo cyclizations in intermediates 5 and 6, respectively. This chemical reaction mimics the direct hydrolysis mechanism of epoxide hydrolases, enzymes that catalyze an epoxide-opening reaction to finally produce vicinal diols.
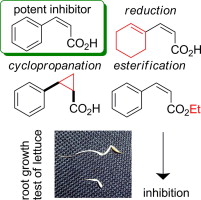
"cis-Cinnamic Acid Selective Suppressors Distinct from Auxin Inhibitors" K. Okuda, K. Nishikawa, H. Fukuda, Y. Fujii, and M. Shindo Chem. Pharm. Bull. 62, 600–607 (2014) [ Open Access ].
ABSTRACT: The activity of cis-cinnamic acid (cis-CA), one of the allelochemicals, in plants is very similar to that of indole-3-acetic acid (IAA), a natural auxin, and thus cis-CA has long been believed to be an analog of auxin. We have reported some
structure–activity relationships studies by synthesizing over 250 cis-CA derivatives and estimating their inhibitory activities on root growth
inhibition in lettuce. In this study, the compounds that showed low- or
no-activity on root growth inhibition were recruited as candidates suppressors
against cis-CA and/or auxin and tested for their activity. In the presence of cis-CA, lettuce root growth was inhibited; however, the addition of some cis-CA derivatives restored control-level root growth. Four compounds, (Z)-3-(4-isopropylphenyl)acrylic acid, (Z)-3-(3-butoxyphenyl)acrylic acid, (Z)-3-[3-(pentyloxy)phenyl]acrylic acid, and (Z)-3-(naphthalen-1-yl)acrylic acid were selected as candidates for a cis-CA selective suppressor they allowed the recovery of root growth from
inhibition by cis-CA treatment without any effects on the IAA-induced effect or elongating
activity by themselves. Three candidates significantly ameliorated the
root shortening by the potent inhibitor derived from cis-CA. In brief, we have found some cis-CA selective suppressors which have never been reported from inactive
cis-CA derivatives for root growth inhibition. cis-CA selective suppressors will play an important role in elucidating the
mechanism of plant growth regulation.
"Transcriptomic evaluation of the enhanced plant growth-inhibitory
activity caused by derivatization of cis-cinnamic acid" N. Wasano,
M. Sugano, K. Nishikawa, K. Okuda, M. Shindo, S.-Y. Park, S. Hiradate,
T. Kamo, and Y. Fujii J. Pestic. Sci. 39, 85–90 (2014 ) [ Open Access ].
ABSTRACT: To establish a rapid high-throughput evaluation system for the enhanced plant growth-inhibitory activity caused by modifications of cis-cinnamic acid’s (cis-CA’s) chemical structure, a DNA microarray assay was used to analyze the
changes in early gene responses of Arabidopsis thaliana seedlings. After
a 6-hr exposure to (Z)-3-(3-iodophenyl)acrylic acid, we observed an upregulation in three classes
of early auxin-responsive genes, which was similar to the transcriptional
response to indole-3-acetic acid (IAA), together with an upregulation of
the genes related to environmental stress and toxin detoxification responses.
Gene responses to 2-(3,4-dihydronaphthalen-1-yl)acetic acid were similar
to those to IAA. In contrast, fewer genes were upregulated in response
to its double-bond isomer, (Z)-2-[3,4-dihydronaphthalen-1(2H)-ylidene]acetic acid, than to cis-CA. DNA microarray data suggest that the structurally different cis-CA analogues trigger diverse gene responses.
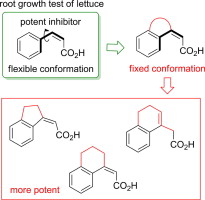
"Design and Synthesis of Conformationally Constrained Analogues of
cis-Cinnamic Acid and Evaluation of Their Plant Growth Inhibitory Activity"
K. Nishikawa, H. Fukuda, M. Abe, K. Nakanishi, Y. Tazawa, C. Yamaguchi,
S. Hiradate, Y. Fujii, K. Okuda, and M. Shindo Phytochemistry 96, 223-234 (2013).
ABSTEACT: 1-O-cis-Cinnamoyl-β-D-glucopyranose is known to be one of the most potent allelochemical candidates
and was isolated from Spiraea thunbergii Sieb by Hiradate et al. (2004), who suggested that it derived its strong inhibitory
activity from cis-cinnamic acid, which is crucial for phytotoxicity. In this study, key structural features and substituent effects of cis-cinnamic acid (cis-CA) on lettuce root growth inhibition was investigated. These structure–activity
relationship studies indicated the importance of the spatial relationship
of the aromatic ring and carboxylic acid moieties. In this context, conformationally
constrained cis-CA analogues, in which the aromatic ring and cis-olefin were connected by a carbon bridge, were designed, synthesized,
and evaluated as plant growth inhibitors. The results of the present study
demonstrated that the inhibitory activities of the five-membered and six-membered
bridged compounds were enhanced, up to 0.27 μM, and were ten times higher
than cis-CA, while the potency of the other compounds was reduced.
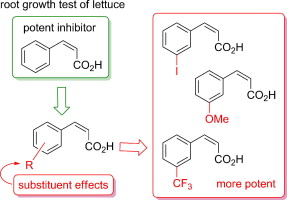
"Substituent Effects of cis-Cinnamic Acid Analogues as Plant Growh Inhibitors" K. Nishikawa,
H. Fukuda, M. Abe, K. Nakanishi, T. Taniguchi, T. Nomura, C. Yamaguchi,
S. Hiradate, Y. Fujii, K. Okuda, and M. Shindo Phytochemistry 96, 132-147 (2013).
1-O-cis-Cinnamoyl-β-D-glucopyranose is one of the most potent allelochemicals that has been
isolated from Spiraea thunbergii Sieb by Hiradate et al. It derives its
strong inhibitory activity from cis-cinnamic acid (cis-CA), which is crucial for phytotoxicity. By preparing and assaying a series
of cis-CA analogues, it was previously found that the key features of cis-CA for lettuce root growth inhibition are a phenyl ring, cis-configuration of the alkene moiety, and carboxylic acid. On the basis of a structure–activity relationship study, the substituent effects on the aromatic ring of cis-CA were examined by systematic synthesis and the lettuce root growth inhibition
assay of a series of cis-CA analogues having substituents on the aromatic ring. While ortho- and para-substituted analogues exhibited low potency in most cases, meta-substitution was not critical for potency, and analogues having a hydrophobic and sterically small substituent were more likely to be potent. Finally, several cis-CA analogues were found to be more potent root growth inhibitors than cis-CA.
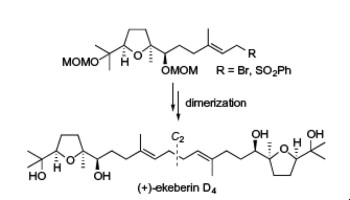
"A Convergent Total Synthesis of Antiplasmodial C2 Symmetric (+)-Ekeberin D4" T. Kodama, S. Aoki, S. Kikuchi, T. Matsuo, Y. Tachi, K. Nishikawa,
and Y. Morimoto Tetrahedron Lett. 54, 5647-5649 (2013).
ABSTRACT: The first concise total synthesis of C2 symmetric (+)-ekeberin D4 (1) that exhibits antiplasmodial activity has been achieved in total nine steps and 27% yield from the known diol 4. The efficient synthetic method features the regio- and diastereoselective
epoxidation of 4 and convergent coupling between half fragments 2 and 3 by taking into account the C2 symmetric property.
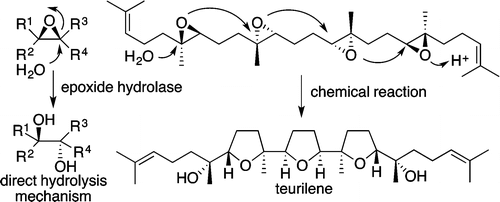
"Biomimetic Epoxide-Opening Cascades of Oxasqualenoids Triggered by
Hydrolysis of the Terminal Epoxide" Y. Morimoto, E. Takeuchi, H. Kambara,
T. Kodama, Y. Tachi, and K. Nishikawa Org. Lett. 15, 2966–2969 (2013).
ABSTRACT: The biomimetic epoxide-opening cascades from squalene polyepoxides 4–6 to triterpene polyethers (oxasqualenoids) teurilene (1), glabrescol (2), and omaezakianol (3), respectively, were reproduced in a single event by chemical reaction.
These cascades proceeded through the 5-exo tandem cyclization triggered by Brønsted acid-catalyzed hydrolysis of
the terminal epoxide, mimicking the direct hydrolysis mechanism of epoxide
hydrolases.
"Root-Specific Induction of Early Auxin-Responsive Genes in Arabidopsis thaliana by cis-Cinnamic Acid" N. Wasano, M. Sugano, K. Nishikawa, K. Okuda, M. Shindo,
H. Abe, S.-Y. Park, S. Hiradate, T. Kamo, and Y. Fujii Plant Biotechnol. 30, 465-471 (2013).
ABSTRACT: cis-Cinnamoyl glucosides are the allelochemicals in Thunberg’s meadowsweet
(Spiraea thunbergii). The essential chemical structure responsible for the bioactivity of
cis-cinnamoyl glucosides, cis-cinnamic acid (cis-CA), strongly inhibits the root growth of several plant species; however,
its mode of action has not been characterized at the gene expression level.
We conducted a time–course microarray analysis of gene expression in Arabidopsis
in response to 20 µM cis-CA. Comparison of the microarray profiles revealed a 10-fold upregulation of several auxin-responsive GRETCHEN HAGEN-3 (GH3) genes and LATERAL ORGAN BOUNDARIES DOMAIN/ASYMMETRIC LEAVES2-LIKE (LBD) genes from 2 h to 6 h post-treatment. Two early auxin-responsive gene
families, the Aux/IAA family (IAA1, IAA5) and the GH3 family (GH3.1, GH3.2, GH3.3), and an LBD gene (LBD16) were markedly upregulated at 2 h after treatment in the roots, but not
in the shoots, of Arabidopsis and remained highly expressed for 4 h. The
influence of an exogenous application of cis-CA on the indole-3-acetic acid pathway strongly suggests that a root-targeted
induction of auxin-responsive genes is involved in the cis-CA-mediated plant growth inhibition.
"Key Structural Features of cis-Cinnamic Acid as an Allelochemical"
M. Abe, K. Nishikawa, H. Fukuda, K. Nakanishi, Y.Tazawa, T. Taniguchi,
S.-Y. Park, S. Hiradate, Y. Fujii, K. Okuda, and M. Shindo Phytochemistry 84, 56-67 (2012).
ABSTRACT: 1-O-cis-cinnamoyl-β-D-glucopyranose is one of the most potent allelochemicals isolated from Spiraea thunbergii Sieb. It is suggested that it derives its strong inhibitory activity from cis-cinnamic acid, which is crucial for phytotoxicity. It was synthesized
to confirm its structure and bioactivity, and also a series of cis-cinnamic acid analogues were prepared to elucidate the key features of
cis-cinnamic acid for lettuce root growth inhibition. The cis-cyclopropyl analogue showed potent inhibitory activity while the saturated
and alkyne analogues proved to be inactive, demonstrating the importance
of the cis-double bond. Moreover, the aromatic ring could not be replaced with a
saturated ring. However, the 1,3-dienylcyclohexene analogue showed strong
activity. These results suggest that the geometry of the C–C double bond
between the carboxyl group and the aromatic ring is essential for potent
inhibitory activity. In addition, using several light sources, the photostability
of the cinnamic acid derivatives and the role of the C–C double bond were
also investigated.
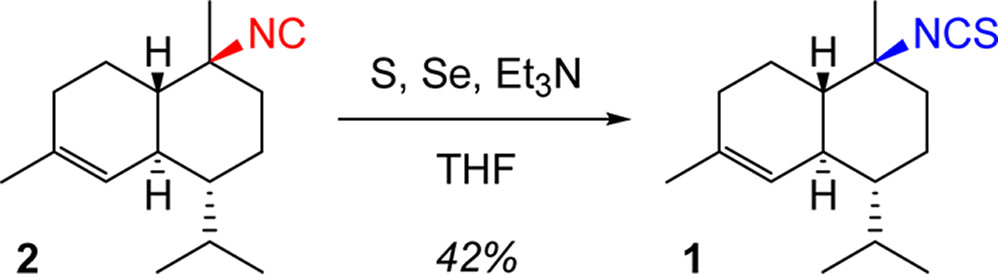
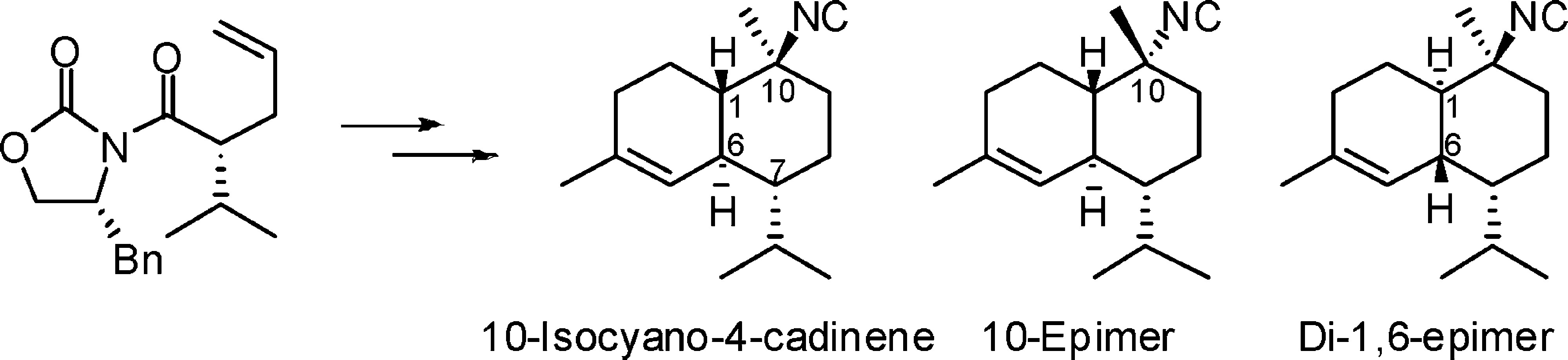
"Confirmation of the Configuration of 10-Isothiocyanato-4-Cadinene
Diastereomers through Synthesis" K. Nishikawa, T. Umezawa, M. J. Garson,
and F. Matsuda J. Nat. Prod. 75, 2232–2235 (2012).
ABSTRACT: The marine sponge metabolite 10-isothiocyanato-4-cadinene (1) was first isolated by Garson et al. from Acanthella cavernosa in 2000. The same structure 1 was later reported by Wright et al. from the nudibranch Phyllidiella pustulosa and its sponge diet, but with different NMR data. The syntheses of both
enantiomers of 1 were accomplished through the isothiocyanation of 10-isocyano-4-cadinene (2) previously synthesized by our group. The correct spectroscopic data and
specific rotation value of the structure 1 were determined on the basis of the syntheses. The NMR data of synthetic 1 matched those of the isothiocyanate isolated by Garson and differed from those reported by Wright. The spectroscopic data and specific rotation values of 10-epi-10-isothiocyanato-4-cadinene (6) and di-1,6-epi-10-isothiocyanato-4-cadinene (8) were also established through the syntheses of these diastereomers. Structure 6 has been reported as a natural product by Mitome et al., but the NMR data
for the synthetic sample of 6 differ from those of the natural isolate.
"Stereoselective Synthesis of β-Glycosyl Esters of cis-Cinnamic Acid and Its Derivatives Using Unprotected Glycosyl Donors"
K. Matsuo, K. Nishikawa, and M. Shindo Tetrahedron Lett. 52, 5688-5692 (2011).
ABSTRACT: The β-glycosyl esters of cis-cinnamic acid were synthesized directly using Hannesian’s unprotected
glycosyl donor and the carboxylic acid in toluene. This protocol does not
require protecting groups on the glycosyl donors, and high stereoselectivity
was achieved. The first synthesis of a potent allelochemical, 1-O-cis-cinnamoyl-β-d-glucopyranose, is also described.
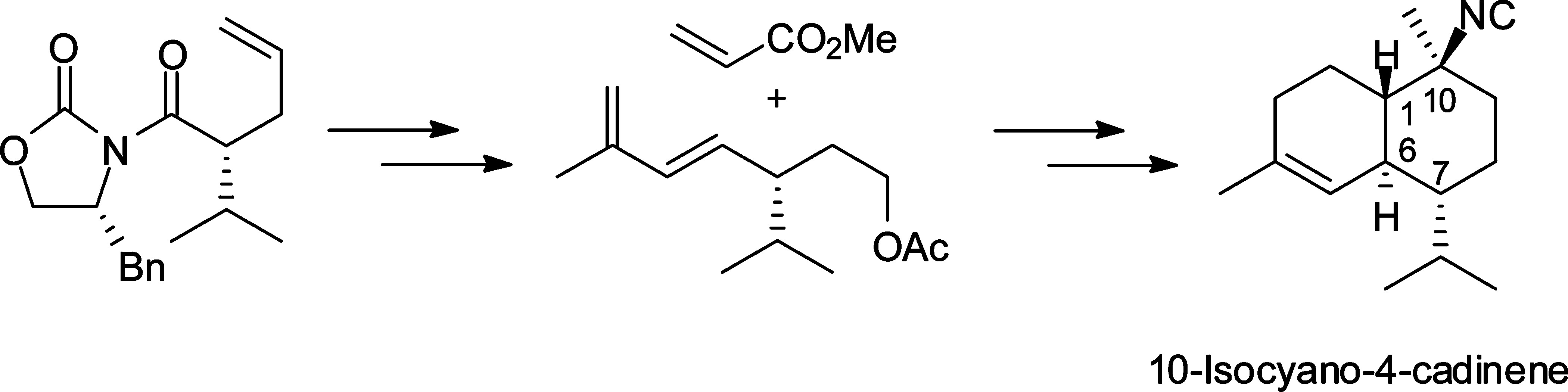
"Total Synthesis of 10-Isocyano-4-Cadinene and Its Stereoisomers and
Evaluations of Antifouling Activities" K. Nishikawa, H. Nakahara,
Y. Shirokura, Y. Nogata, E. Yoshimura, T. Umezawa, T. Okino, and F. Matsuda
J. Org. Chem. 76, 6558–6573 (2011).
ABSTRACT: The first enantioselective total synthesis of 10-isocyano-4-cadinene,
a marine sesquiterpene isolated from nudibranchs of the family Phyllidiidae, and determination of its absolute stereochemistry were achieved. 10-Isocyano-4-cadinene
is expected to be a novel nontoxic antifouling agent. In the synthesis,
intermolecular Diels–Alder reaction and samarium diiodide induced Barbier-type
cyclization were employed as key steps. The absolute configuration of 10-isocyano-4-cadinene
was determined as (1S,6S,7R,10S) by comparison of the optical rotations between natural and synthetic samples. In addition, the authors successfully synthesized 10-epi- and di-1,6-epi-10-isocyano-4-cadinene through the same synthetic pathway. Antifouling
activities against Balanus amphitrite with the cadinenes were also evaluated.
"Total Synthesis of 10-Isocyano-4-cadinene and Determination of Its
Absolute Configuration" K. Nishikawa, H. Nakahara, Y. Shirokura, Y.
Nogata, E. Yoshimura, T. Umezawa, T. Okino, and F. Matsuda Org. Lett. 12, 904–907 (2010).
ABSTRACT: The first enantioselective total synthesis of 10-isocyano-4-cadinene,
a marine sesquiterpene isolated from nudibranchs of the family Phyllidiidae, was achieved. The cadinene is expected to be a novel nontoxic antifouling
agent. In the synthesis, an intermolecular Diels−Alder reaction and a SmI2-induced Barbier-type reaction were employed as key steps. The absolute
configuration of 10-isocyano-4-cadinene was determined to be (1S, 6S, 7R, 10S) on the basis of the total synthesis. Antifouling activities against Balanus amphitrite with both enantiomers of 10-isocyano-4-cadinene were also evaluated.
まだ工事中です。